Dr. Fratazzi’s contributions have been significant. Her journey began when she founded BBCR Consulting, an organization dedicated to strategic clinical innovation, aiming to streamline the often convoluted processes of clinical trials. Drawing from her extensive 25 years in biomedical research, Dr. Fratazzi introduced the SCIOTM concept, a revolutionary approach aimed at creating efficiencies in the […]

Her journey began when she founded BBCR Consulting, an organization dedicated to strategic clinical innovation, aiming to streamline the often convoluted processes of clinical trials. Drawing from her extensive 25 years in biomedical research, Dr. Fratazzi introduced the SCIOTM concept, a revolutionary approach aimed at creating efficiencies in the intricate journey of bringing biotech products […]

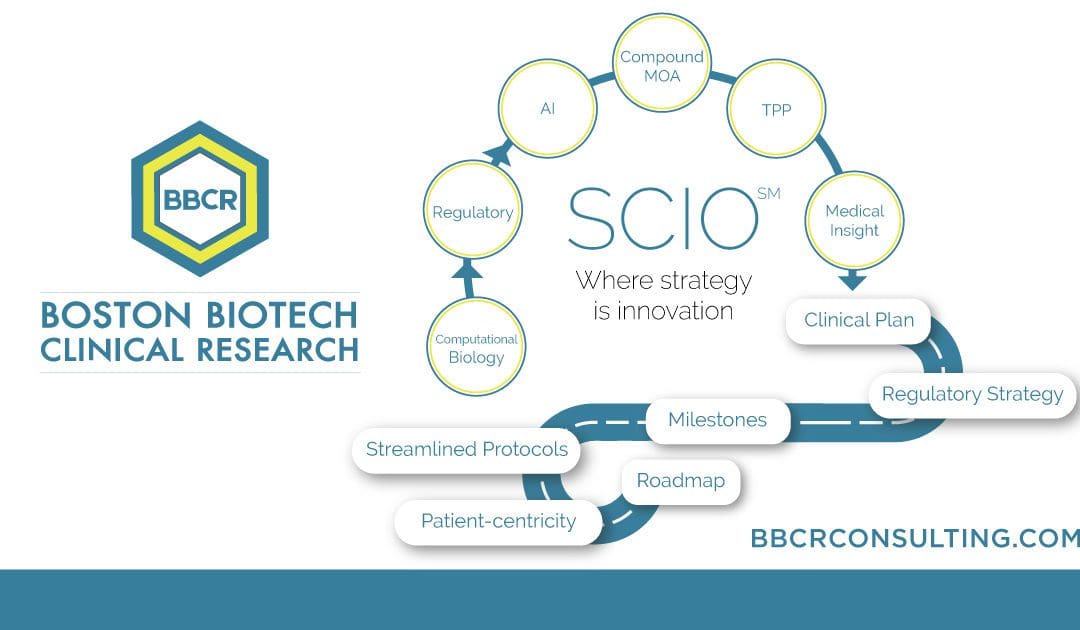
Our selected services include expertise with Biomarkers & Surrogate Endpoints, Regulatory Affairs FDA & EMA, Medical Affairs & Clinical Research, Due Diligence, and Trial Management & Trial Rescue. BBCR’s Strategic Clinical Innovation OrganizationSM (SCIO) method is explicitly designed to help pharmaceutical innovators address their concerns and maneuver around evolving challenges. SCIOSM identifies time and cost […]

Innovation in clinical research is a long-term need due to several factors, including the high failure rate and skyrocketing costs. Healthcare has been changing rapidly for many years. The COVID-19 pandemic has accelerated by helping drive the shift to value-based healthcare. Instead, the productivity of pharmaceutical development has been declining due to high failure rates […]

Innovation in clinical research is a long-term need due to several factors, including the high failure rate and skyrocketing costs. Several factors contribute to the skyrocketing cost of clinical trials. Integration of advanced technologies into drug development. While these technologies offer valuable insights, they incur additional training, data analysis, and interpretation costs. Complexity of study […]

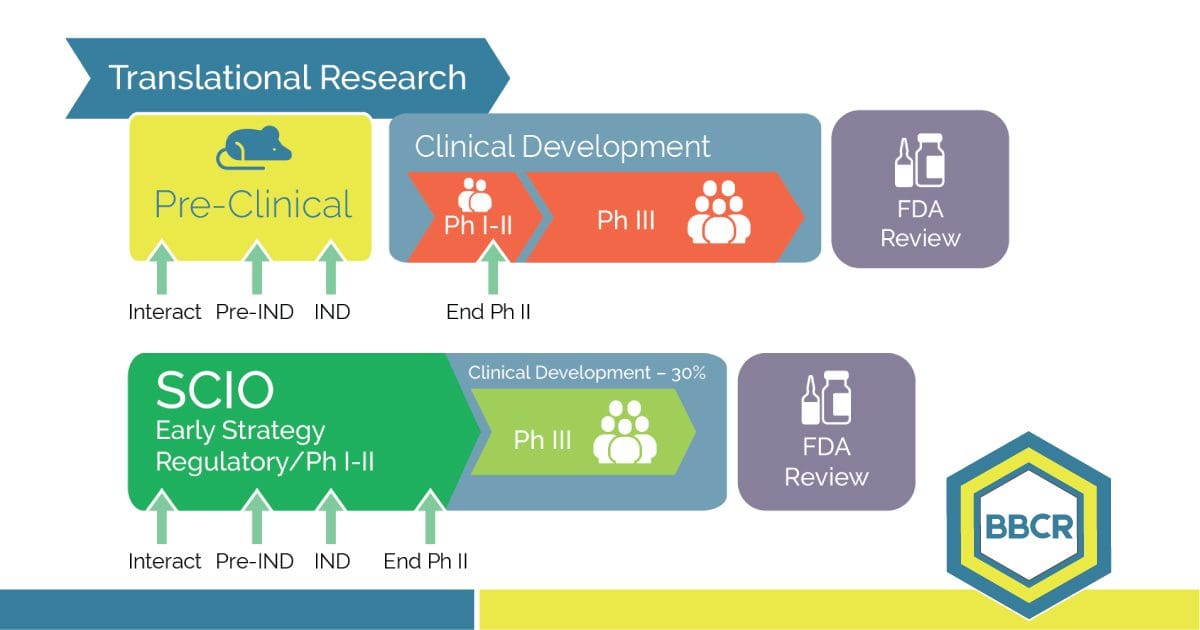
An early-phase strategy improves productivity and the path to market approval. SCIO SM Advantages Accelerate Patient Recruitment Reduce Patient Number Reduce Clinical Development Time Reduce Trial Monitoring Time Increase Patient Retention Facilitate Decision Making Increase Data Quality The FDA has been calling for a smarter, more innovative process for market approval, and SCIO SM is […]

Learn the potentials of each preclinical asset, and predict the level of certainty for each strategic option. Today’s technological molecules require innovation in the development process. We cannot keep using yesterday’s assumptions for tomorrow’s drugs. Marketed product evidence proves that identical molecules developed by different sponsors generated very different drug opportunities. Data show that options’ […]

The Strategic Clinical Innovation Organization SCIO SM (SCIO) method is explicitly designed to help pharmaceutical innovators address their concerns and maneuver around evolving challenges. SCIO SM allows for time and cost efficiencies and relief of risk management. The SCIO SM method aims to learn, predict and make better decisions for a successful drug opportunity. This […]
The Trial Management That Addresses Risks Experienced management address risks such as: Delayed enrollment Multiple protocol amendments Poor patient retention Poor data quality Prolonged trial completion timeline Poor safety monitor Let BBCR Consulting assist in your clinical trial strategy. Reach out to us to learn more about how we can help.
How can SCIO SM help? To enhance your position, it becomes crucial to demonstrate that your product/technology is the “safe” option for investment and a stand-out opportunity by including a clinical roadmap and development strategy of your product/technology. This roadmap illuminates clearly for the investment team what the target indications will be and the paths […]