BBCR Voice
From the Perspective of Researchers, Clinicians, and Regulatory Experts
The most efficient path in the clinical research process is a moving target. Technology innovation and regulatory requirements require constant updates. Through BBCR Voice, we aim to share not only our knowledge and expertise but also solutions to current challenges. BBCR embraces the challenges of developers and investors seeking a more straightforward path to market.
Recent Posts
Accelerate Drug Development with Biomarker Strategy in Orphan Diseases and Precision Medicine
In the fields of orphan diseases and precision medicine, establishing a robust biomarker strategy during translational and early clinical development is key to expediting time-to-market. A well-defined biomarker plan not only streamlines regulatory approval but also...
At BBCR, our team of seasoned experts is dedicated to helping clients navigate the intricate landscape of rare genetic conditions and unsolved diseases. We work hand in hand with product developers to create the most effective strategies for bringing innovative treatments to market.
At BBCR, our team of seasoned experts is dedicated to helping clients navigate the intricate landscape of rare genetic conditions and unsolved diseases. We work hand in hand with product developers to create the most effective strategies for bringing innovative...
Our experienced CRO Management and Drug development team identify study remediation strategies and provide a resource for any Study Rescue. We invite you to reach out to learn more – visit bbcrconsulting.com.
Every clinical study has its unique challenges that initially may not have been factored for. Experienced management can help sponsors to address prolonged trial timeline and high quality data. Rare Diseases and Precision Medicine Require Unique Approaches In Clinical...
A pioneering figure in the area of rare diseases and orphan drug early-stage clinical research and regulatory strategies, Dr. Candida Fratazzi has an impressive 25-year track record in this specialized field.
Dr. Fratazzi's contributions have been significant. Her journey began when she founded BBCR Consulting, an organization dedicated to strategic clinical innovation, aiming to streamline the often convoluted processes of clinical trials. Drawing from her extensive 25...
BBCR is committed to cultivating and enhancing a product’s unique strengths at every stage, from initial conception to successful market launch. With deep expertise in cell therapy, biologics, and gene therapy—particularly in the realm of rare diseases—we provide strategic guidance and innovative solutions that form the foundation of our consultancy practice.
We are dedicated to addressing the complex challenges faced by sponsors and investors who seek a clear and streamlined path to market. Our approach focuses on nurturing the inherent strengths of their products while simultaneously enhancing efficiency, safety, and...
BBCR embraces innovative strategic consulting for highly effective clinical development planning and regulatory strategy. Reach out today to learn more about how we can help advance your project.
Our industry needs innovative strategies, and reduced-risk clinical trials. BBCR clinical development services and drug development consulting integrates real world evidence into clinical development plans and regulatory strategies. We ensure that focus goes towards...
Partnering with BBCR Consulting streamlines the clinical trial process by delivering tailored clinical and regulatory roadmaps. Our approach focuses on simplified programs and optimized protocols, designed to align seamlessly with each client’s unique requirements.
We invite you to read some of our Case Studies below Reduced Comparator High Cost for Biosimilars PROJECT An executive at a pharmaceutical company asked BBCR to review its biosimilar pre-clinical data and prepare the IND package. In addition to evaluate the...
BBCR meets clients’ needs in the fast paced and ever-changing regulatory environment. As specialists in Orphan and Personalized Medicine, BBCR helps clients identify areas of need or economic interest and helps them to find homes for treatments for rare diseases and precision medicine.
BBCR helps orphan drug developers find direction in clinical trials involving biologics, biosimilars, small molecules, medical devices, and repurposing. BBCR consultants have the experience to guide you through the development process with a clinical plan and a...
BBCR is highly experienced in developing innovative approaches to de-risk your product development during the early clinical development stage, including designing Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies. Learn more at bbcrconsulting.com #PoC #PoM #proofofconcept #proofofmechanism #earlyclinicaldevelopment #strategy
BBCR specializes in the strategy and delivery of early-phase clinical development services to enable informed, timely decision making for our clients. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose...
BBCR partners with small and medium sized drug and device biotechnology firms. Our clients are clinical researchers and innovators looking for an efficient path to approval, and come to us from across the globe. Learn more about our consulting services at bbcrconsulting.com.
The BBCR mission is to simplify clinical research, encourage cost-effective trials, and help innovators navigate through the regulatory process. Who We Serve: Small & Medium Sized Biotech Companies currently moving from pre-clinical studies toward clinical trials...
From Preclinical through Phase I and POC studies, BBCR provides early clinical research services to enable informed, timely decision-making for our clients. We offer clinical, regulatory, translational, and biomarker consulting services that support our clients’ needs. Our process is designed to maximize time and cost efficiencies while mitigating risk.
BBCR Consulting offers clinical, regulatory, translational, and biomarker consulting services that support our clients’ needs. Our process is designed to maximize time and cost efficiencies while mitigating risk. Our consultants have the experience to guide you...
With Biomarkers a routine part of drug development, BBCR assists companies with the identification and adoption of biomarkers, especially valuable in rare disease and precision medicine product development.
The FDA recognizes biomarker development as a high priority area for future research. BBCR can help your company with your product development plan and validation. BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan...
“The beginning is the most important part of the work.” — Plato. BBCR is dedicated to developing and nurturing a product’s unique strengths from conception to market. Our expertise with cell, biologics and gene therapy as they relate to rare disease is the cornerstone of our consultancy practice.
BBCR is committed to meeting the challenges of sponsors and investors seeking a clear path to market by nurturing their products’ strengths while improving efficiency and safety. We specialize in strategy and provide early clinical research services to enable...
Dr. Candida Fratazzi is a pioneering figure in the area of rare diseases and orphan drug early-stage clinical research and regulatory strategies. With an impressive 20-year track record in this specialized field, Dr. Fratazzi’s contributions have been significant.
Her journey began when she founded BBCR Consulting, an organization dedicated to strategic clinical innovation, aiming to streamline the often convoluted processes of clinical trials. Drawing from her extensive 25 years in biomedical research, Dr. Fratazzi introduced...
BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan drug indications by developing and nurturing the product’s unique strengths. Our operational mission is to craft customized strategies that achieve cost-effective trials. Reach out today to learn more.
BBCR’s mission is to deliver cost-effective clinical plans and regulatory strategies for cell and gene therapy programs in the areas of rare disease. Cell and Gene therapy are the new frontiers in the fight against devastating diseases, including rare diseases and...
BBCR provides Orphan Drug solutions that empower sponsors with medical insight, ODA application experience, strategic clinical planning, and streamlined trial design.
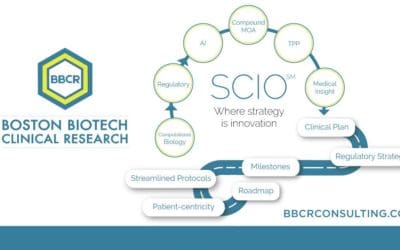
Our selected services include expertise with Biomarkers & Surrogate Endpoints, Regulatory Affairs FDA & EMA, Medical Affairs & Clinical Research, Due Diligence, and Trial Management & Trial Rescue. BBCR's Strategic Clinical Innovation OrganizationSM...
By collaborating with Boston Biotech Clinical Research, you’ll be working with a team of experts who are dedicated to streamlining the clinical trial process.
We provide expert guidance for Orphan Drug Development by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. BBCR is dedicated to supporting pharmaceutical innovators in the specialized...
BBCR’s mission is to deliver cost-effective clinical plans and regulatory strategies for cell and gene therapy programs in the areas of rare disease. We invite you to learn more at bbcrconsulting.com.
Cell and Gene therapy are the new frontiers in the fight against devastating diseases, including rare diseases and cancers. Clinical trial results have been promising, and the next generation of Cell and Gene therapies holds tremendous promise for many patients and...
BBCR uses innovative approaches to de-risk your product development. For our clients interested in Proof of Concept and Proof of Mechanism – PoC and PoM – Our team has the expertise to ensure successful product development at any stage of development.
The BBCR team designs Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies with the drug clinical plan and regulatory strategy in mind. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose...
Why a Strategy Plan Before First in Human
Innovation in clinical research is a long-term need due to several factors, including the high failure rate and skyrocketing costs. Healthcare has been changing rapidly for many years. The COVID-19 pandemic has accelerated by helping drive the shift to value-based...
Why and how to invest in Early Strategy before First in Human
Innovation in clinical research is a long-term need due to several factors, including the high failure rate and skyrocketing costs. Several factors contribute to the skyrocketing cost of clinical trials. Integration of advanced technologies into drug development....
BBCR is committed to meeting the challenges of sponsors and investors seeking a clear path to market.
Our mission is to support domestic and international pharma, biotech, and device companies by nurturing their products’ strengths while improving efficiency and safety. We specialize in strategy and provide early clinical research services to enable informed, timely...
There remains continued interest in the use of surrogate endpoints as primary measures of the effectiveness of investigational drugs in definitive drug trials. BBCR’s published White Paper – Surrogate Endpoints for an Accelerated Orphan Drug Approval – remains a valuable resource in the area of orphan drug development.
Rare Diseases are conditions that affect less than 200,000 people in the U.S. However, over 7,000 Rare Diseases affect more than 400,000,000 people worldwide, including ~25 million in the U.S. and ~30 million in Europe (1). In recent years, the number of orphan drugs...
Early Clinical Strategy Mistakes to Avoid
While an early clinical strategy offers numerous advantages, it also comes with certain challenges that need to be considered: Limited Information: Limited information available about the investigational product can make it challenging. High Risk of Failure:...
An Early Clinical Strategy Includes Several Key Elements
Developing an effective early clinical strategy requires collaboration between various experts. The strategy is a living document that can evolve as new data becomes available and as the project progresses through clinical development. Study Design: The design must be...