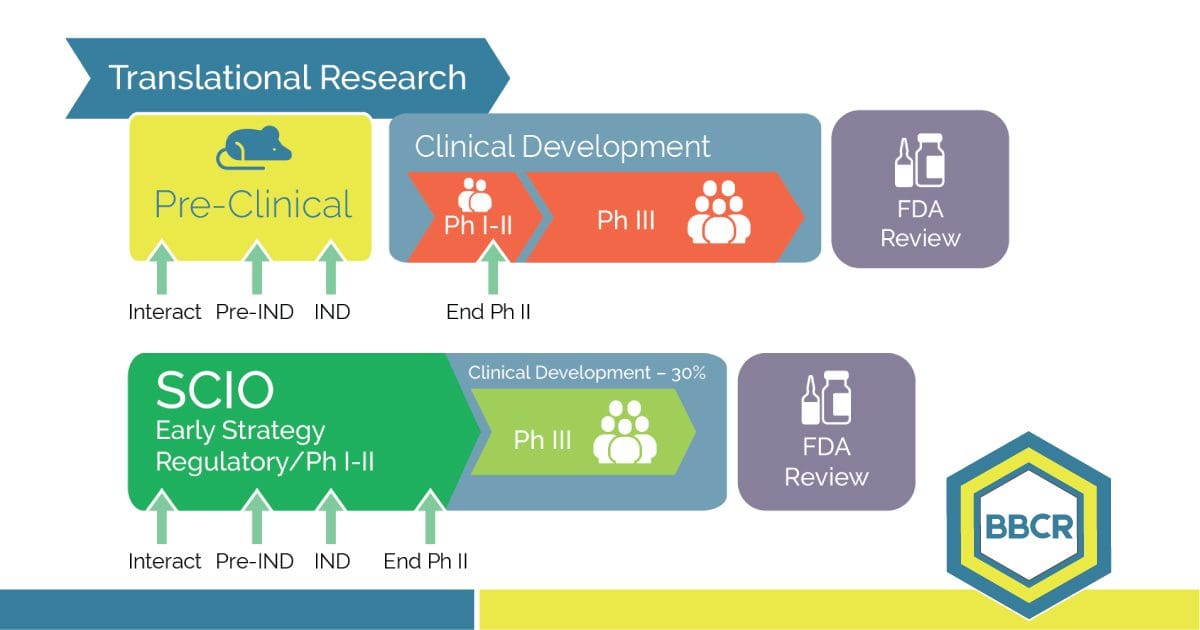
Our industry needs innovative strategies, and reduced-risk clinical trials. BBCR clinical development services and drug development consulting integrates real world evidence into clinical development plans and regulatory strategies. We ensure that focus goes towards cost containment and value-based developments that allow sponsors to move more treatments to market faster. BBCR’s team expertise in rare diseases […]

BBCR helps orphan drug developers find direction in clinical trials involving biologics, biosimilars, small molecules, medical devices, and repurposing. BBCR consultants have the experience to guide you through the development process with a clinical plan and a regulatory strategy. Our team will empower you with medical insight, effective regulatory expertise, strategy, and streamlined early clinical […]

Expanding a product portfolio through the selection of additional indications can offer strategic growth opportunities. However, the process of selecting indications for portfolio expansion requires careful consideration of various factors. Here are few of them for your consideration: Synergy with Existing Capabilities: Building on established strengths can streamline development and increase the chances of success. […]

The FDA recognizes biomarker development as a high priority area for future research. BBCR can help your company with your product development plan and validation.

Translational medicine is a bridge between pre-clinical and early clinical development (from bench to bedside), creating the foundation for precision medicine in late clinical development. A translational medicine program includes the adopting biomarkers to mitigate clinical trial risk, identify patient subpopulations, facilitate decision-making, and accelerate a drug’s market approval.

Learn the potentials of each preclinical asset, and predict the level of certainty for each strategic option. Today’s technological molecules require innovation in the development process. We cannot keep using yesterday’s assumptions for tomorrow’s drugs. Marketed product evidence proves that identical molecules developed by different sponsors generated very different drug opportunities. Data show that options’ […]

Real-world evidence (RWE) is increasingly important in clinical trial design and drug approval. RWE is clinical evidence related to patient health status and treatment safety and efficacy that are generated in clinical practice. BBCR’s team understands the role RWE plays for Natural History in Rare Diseases. We invite you to be in touch to learn […]

Increase investors’ evaluation in a competitive market. Due Diligence is typically performed by investors but can, and should, be conducted by sponsors to increase their chance of financing success, partnering, mergers, acquisitions (M&As), and product licensing. For companies developing multiple products, due Diligence can also inform and assist in deciding which products to budget for […]

The BBCR team designs Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies with the drug clinical plan and regulatory strategy in mind. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose for PoC, disease model and biomarkers Investigate drug concentration at the target […]
Translational medicine is a bridge between pre-clinical and early clinical development (from bench to bedside), creating the foundation for precision medicine in late clinical development. A translational medicine program includes the adoption of biomarkers to mitigate clinical trials risk, identify patient subpopulations, facilitate decision-making, and accelerate drugs’ market approval.