In the fields of orphan diseases and precision medicine, establishing a robust biomarker strategy during translational and early clinical development is key to expediting time-to-market. A well-defined biomarker plan not only streamlines regulatory approval but also aligns with FDA expectations for safety and efficacy. Discover how our expertise in biomarkers and surrogate endpoints can help […]

BBCR Consulting offers clinical, regulatory, translational, and biomarker consulting services that support our clients’ needs. Our process is designed to maximize time and cost efficiencies while mitigating risk. Our consultants have the experience to guide you through the Orphan clinical research process with a clinical plan and regulatory strategy for accelerated approval. Our team will […]

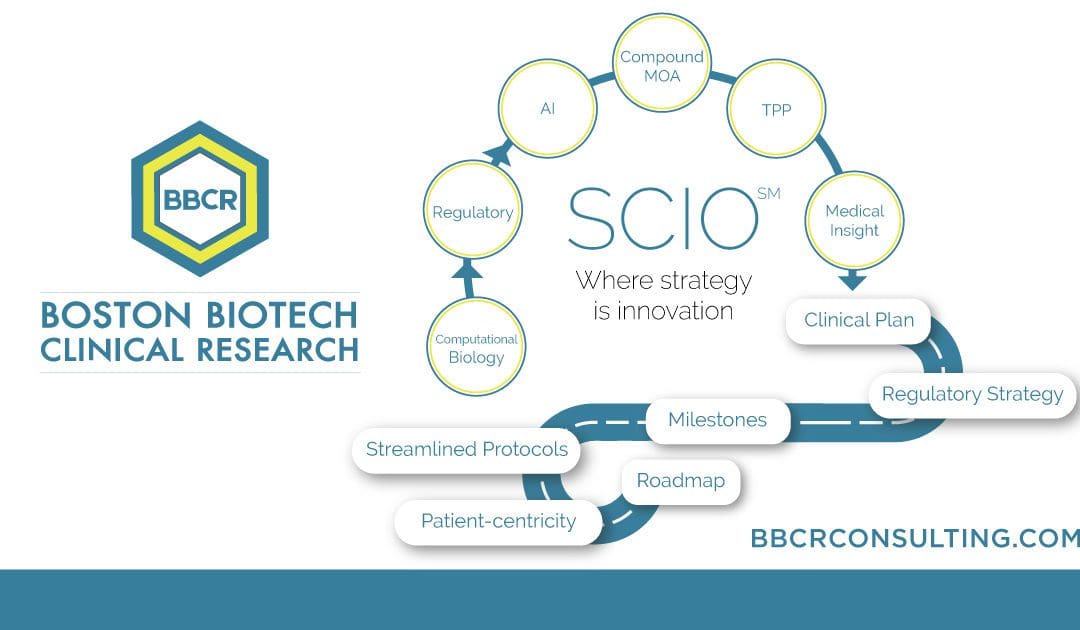
Our selected services include expertise with Biomarkers & Surrogate Endpoints, Regulatory Affairs FDA & EMA, Medical Affairs & Clinical Research, Due Diligence, and Trial Management & Trial Rescue. BBCR’s Strategic Clinical Innovation OrganizationSM (SCIO) method is explicitly designed to help pharmaceutical innovators address their concerns and maneuver around evolving challenges. SCIOSM identifies time and cost […]

We provide expert guidance for Orphan Drug Development by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan drug indications by developing and nurturing the product’s unique strengths. Our operational mission is […]

Innovation in clinical research is a long-term need due to several factors, including the high failure rate and skyrocketing costs. Several factors contribute to the skyrocketing cost of clinical trials. Integration of advanced technologies into drug development. While these technologies offer valuable insights, they incur additional training, data analysis, and interpretation costs. Complexity of study […]

Our mission is to support domestic and international pharma, biotech, and device companies by nurturing their products’ strengths while improving efficiency and safety. We specialize in strategy and provide early clinical research services to enable informed, timely decision-making for our clients. Learn more about the services we provide within the areas of Early Clinical Research […]

Rare Diseases are conditions that affect less than 200,000 people in the U.S. However, over 7,000 Rare Diseases affect more than 400,000,000 people worldwide, including ~25 million in the U.S. and ~30 million in Europe (1). In recent years, the number of orphan drugs approved has increased significantly. However, developing and marketing orphan drugs remains […]

Expanding a product portfolio through the selection of additional indications can offer strategic growth opportunities. However, the process of selecting indications for portfolio expansion requires careful consideration of various factors. Here are few of them for your consideration: Synergy with Existing Capabilities: Building on established strengths can streamline development and increase the chances of success. […]

The BBCR team will empower Sponsors with medical insight, ODA application experience, strategic clinical planning, and streamlined trial design.

An early-phase strategy improves productivity and the path to market approval. SCIO SM Advantages Accelerate Patient Recruitment Reduce Patient Number Reduce Clinical Development Time Reduce Trial Monitoring Time Increase Patient Retention Facilitate Decision Making Increase Data Quality The FDA has been calling for a smarter, more innovative process for market approval, and SCIO SM is […]