Innovation in clinical research is a long-term need due to several factors, including the high failure rate and skyrocketing costs.
Several factors contribute to the skyrocketing cost of clinical trials.
- Integration of advanced technologies into drug development. While these technologies offer valuable insights, they incur additional training, data analysis, and interpretation costs.
- Complexity of study designs, such as adaptive trial design. These designs require more sophisticated statistical analyses, monitoring, and regulatory oversight, contributing to increased costs.
- Regulatory bodies more stringent requirements. Compliance with these regulations increases the costs of trial design, documentation, monitoring, and reporting.
- Cost of investigational products. Developing and manufacturing investigational products, including drugs, biologics, and medical devices, has risen significantly.
- Treating chronic conditions. These indications have multifactorial causes and present heterogeneity among patients. Understanding the underlying mechanisms, large sample sizes to demonstrate the statistical significance and adequately represent diverse patient populations, recruiting and retaining many participants, and longitudinal follow-up to assess long-term safety and efficacy outcomes in chronic conditions contribute to the overall cost of clinical trials.
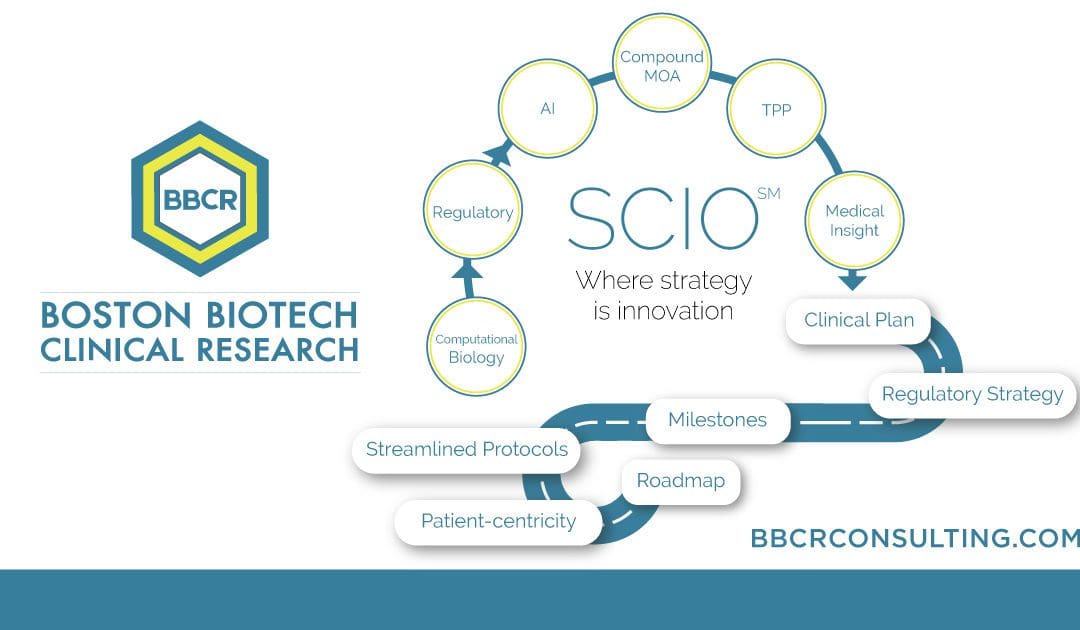
An Early Strategy mitigates risks, optimizes trial design and execution, and increases the chances of successful outcomes. The SCIO method hinges on developing product-specific early strategies with innovative practices that overcome the challenges associated with traditional clinical development. Leveraging artificial intelligence (AI), data analytics, and a deep understanding of regulatory affairs and medical knowledge to streamline processes, enhance patient recruitment and engagement, and optimize trial design and execution.
Small molecules, biologics, cell or gene therapies for orphan drugs, or common indications can benefit from a SCIO method strategy plan and roadmap.

Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.