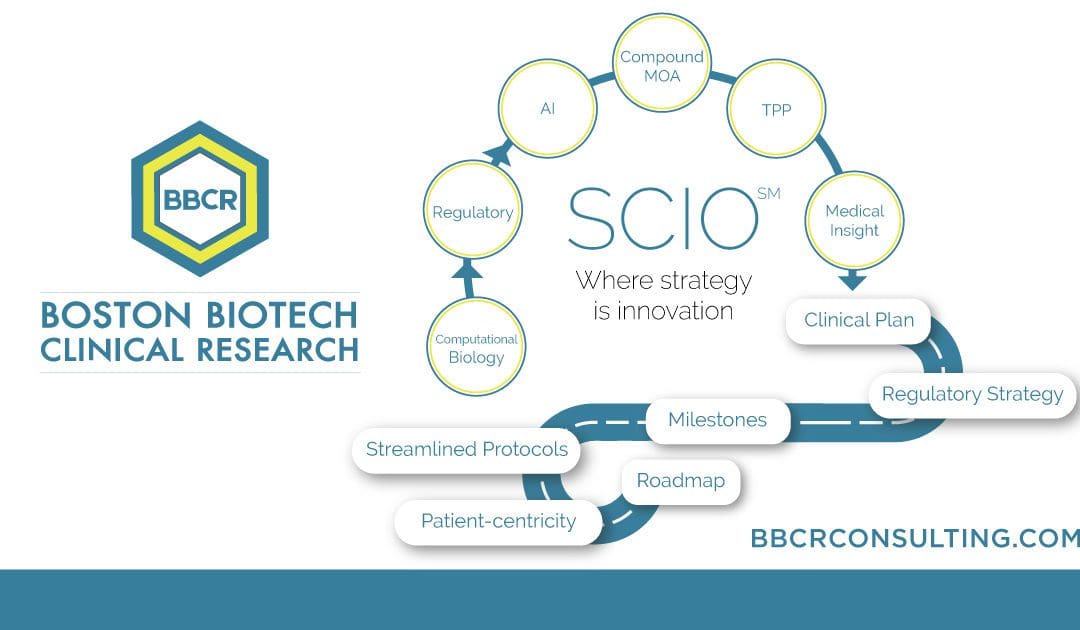
Dr. Fratazzi’s contributions have been significant. Her journey began when she founded BBCR Consulting, an organization dedicated to strategic clinical innovation, aiming to streamline the often convoluted processes of clinical trials. Drawing from her extensive 25 years in biomedical research, Dr. Fratazzi introduced the SCIOTM concept, a revolutionary approach aimed at creating efficiencies in the […]

Our industry needs innovative strategies, and reduced-risk clinical trials. BBCR clinical development services and drug development consulting integrates real world evidence into clinical development plans and regulatory strategies. We ensure that focus goes towards cost containment and value-based developments that allow sponsors to move more treatments to market faster. BBCR’s team expertise in rare diseases […]

BBCR helps orphan drug developers find direction in clinical trials involving biologics, biosimilars, small molecules, medical devices, and repurposing. BBCR consultants have the experience to guide you through the development process with a clinical plan and a regulatory strategy. Our team will empower you with medical insight, effective regulatory expertise, strategy, and streamlined early clinical […]

Her journey began when she founded BBCR Consulting, an organization dedicated to strategic clinical innovation, aiming to streamline the often convoluted processes of clinical trials. Drawing from her extensive 25 years in biomedical research, Dr. Fratazzi introduced the SCIOTM concept, a revolutionary approach aimed at creating efficiencies in the intricate journey of bringing biotech products […]

Our selected services include expertise with Biomarkers & Surrogate Endpoints, Regulatory Affairs FDA & EMA, Medical Affairs & Clinical Research, Due Diligence, and Trial Management & Trial Rescue. BBCR’s Strategic Clinical Innovation OrganizationSM (SCIO) method is explicitly designed to help pharmaceutical innovators address their concerns and maneuver around evolving challenges. SCIOSM identifies time and cost […]

Innovation in clinical research is a long-term need due to several factors, including the high failure rate and skyrocketing costs. Several factors contribute to the skyrocketing cost of clinical trials. Integration of advanced technologies into drug development. While these technologies offer valuable insights, they incur additional training, data analysis, and interpretation costs. Complexity of study […]

Providing Expert Guidance for Orphan Drug Development BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan drug indications by developing and nurturing the product’s unique strengths. Our operational mission is to craft customized strategies that achieve cost-effective trials by 1) simplifying clinical plans, 2) streamlining trial protocols and 3) creating […]

An early-phase strategy improves productivity and the path to market approval. SCIO SM Advantages Accelerate Patient Recruitment Reduce Patient Number Reduce Clinical Development Time Reduce Trial Monitoring Time Increase Patient Retention Facilitate Decision Making Increase Data Quality The FDA has been calling for a smarter, more innovative process for market approval, and SCIO SM is […]

Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. Click the button below to learn more about how we have helped companies with their clinical trial needs.

Streamlining Clinical Development with Clinical and Regulatory Expertise Our team will empower you with medical insight, practical regulatory, translational medicine, and clinical research expertise, and streamlined clinical trials.