Learn the potentials of each preclinical asset, and predict the level of certainty for each strategic option.
Today’s technological molecules require innovation in the development process. We cannot keep using yesterday’s assumptions for tomorrow’s drugs.
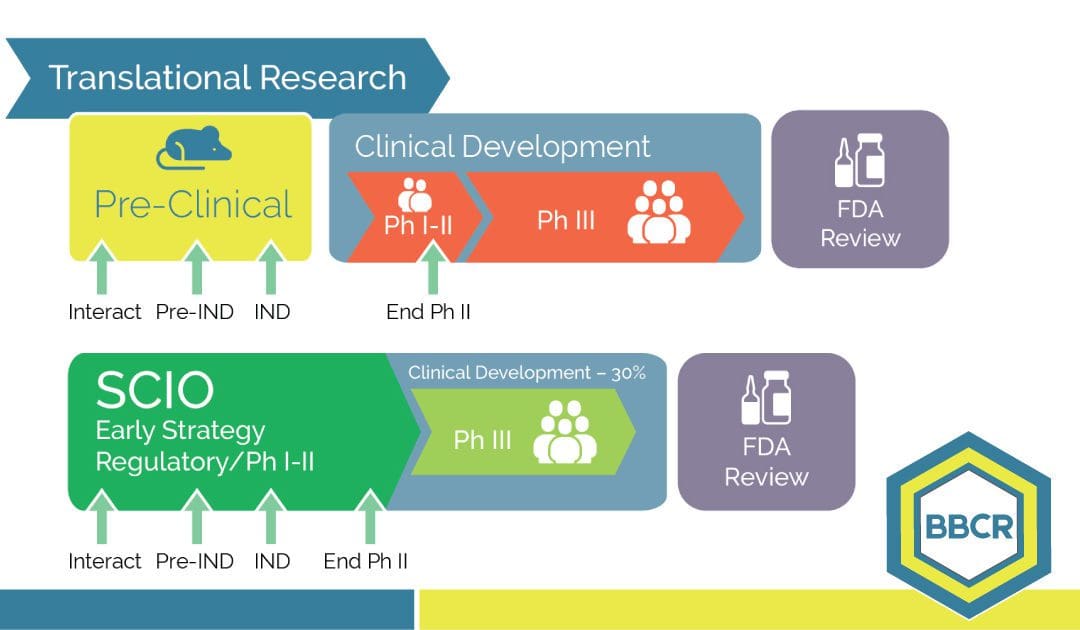
- Marketed product evidence proves that identical molecules developed by different sponsors generated very different drug opportunities.
- Data show that options’ evaluation and early strategy (from indication selection and validation into phase I-II trial) constitute a competitive advantage that a late development redirection of optionality and decisions cannot replace.
Reach out to learn how BBCR can help

Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.