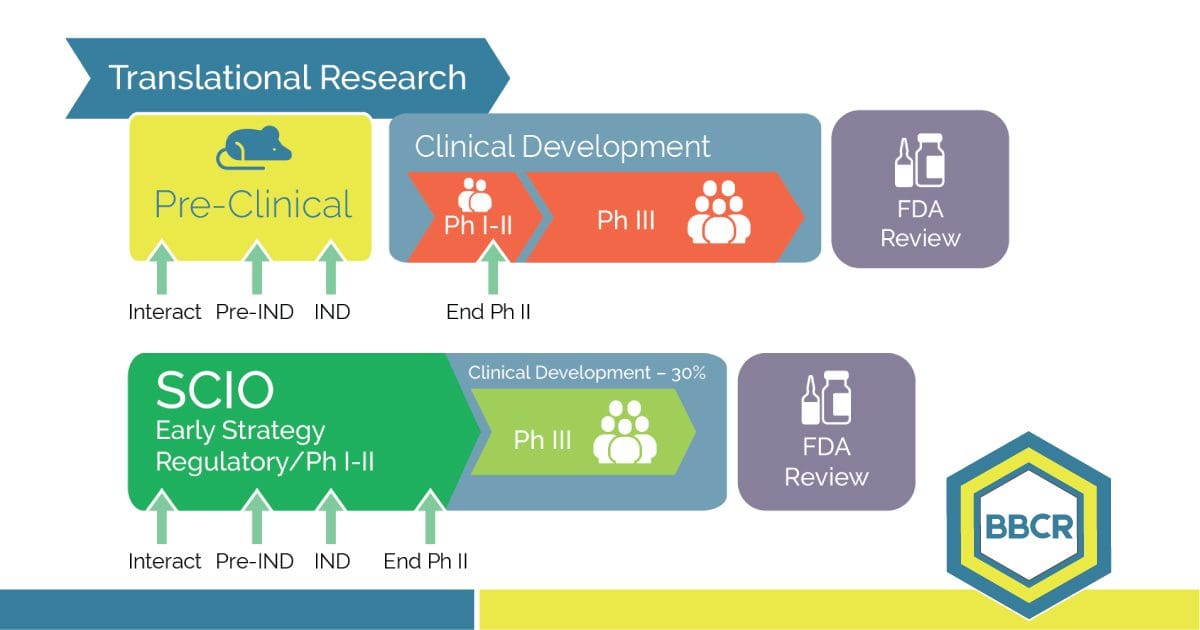
Learn the potentials of each preclinical asset, and predict the level of certainty for each strategic option. Today’s technological molecules require innovation in the development process. We cannot keep using yesterday’s assumptions for tomorrow’s drugs. Marketed product evidence proves that identical molecules developed by different sponsors generated very different drug opportunities. Data show that options’ […]

Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. Click the button below to learn more about how we have helped companies with their clinical trial needs.

Streamlined Clinical Development Strategies Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. […]

Our industry needs innovative strategies, and reduced-risk clinical trials. BBCR clinical development services and drug development consulting integrates real world evidence into clinical development plans and regulatory strategies. Focus must go to cost containment and value-based developments that allow sponsors to move more treatments to market faster. BBCR’s team expertise in rare diseases and precision […]

Biomarkers are considered a routine part of drug development. Learn more about how BBCR can help get your product to market more efficiently with proper plan development and strategy. Plan Development Insightful strategy is the most effective way to ensure quality when including biomarkers into your product development plan. Validation or Qualification Understanding where […]

BBCR Consulting offers world-class regulatory, clinical research, and biomarker consulting services that provide high-value, and support our clients’ operational and functional needs. Our process is designed to maximize time efficiencies, risk mitigation, and cost savings. We partner with domestic and international, small-to-medium drug and device companies that are entering early clinical development. BBCR is dedicated […]