SCIO is critical for accelerating approval and significantly reducing the costs of drug and medical device development. One of the major challenges facing this industry is the rising cost of clinical trials. Thus, it is crucial to ensure that trials are significantly more efficient and cost-effective with the involvement of strategy at the time of […]
Tagged: SCIO
Strategic Consulting and the SCIO Process – Paving the Way for Drug Development
July 19th, 2022 | SCIOSCIO helps early stage biotech company evaluation. Learn why adopting this clinical roadmap and development strategy is critical to your success in bringing your product to market.
April 20th, 2022 | SCIO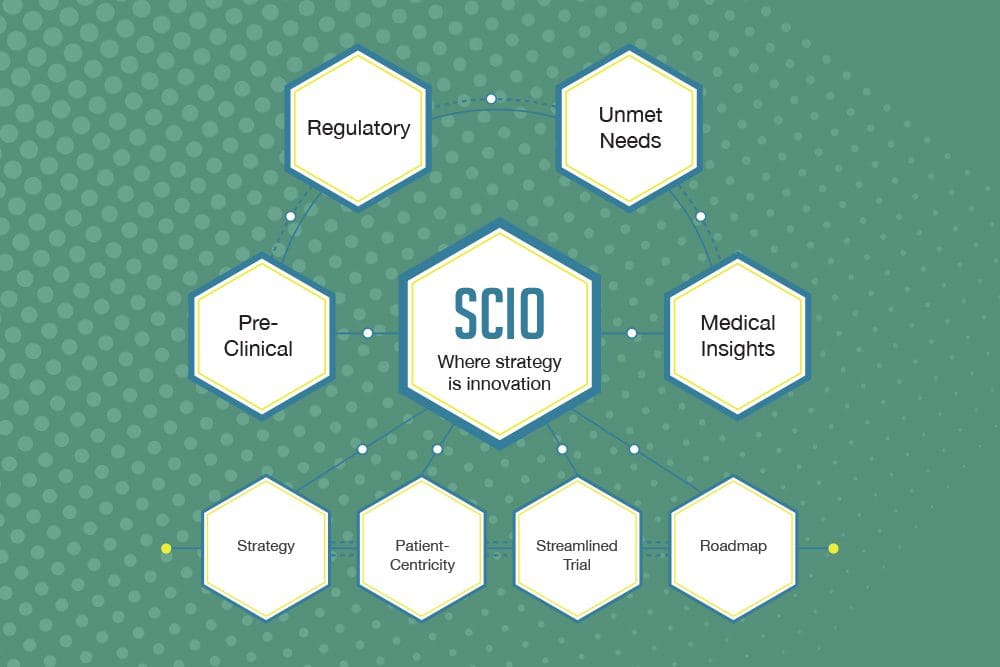
How can SCIO help? To enhance your position, it becomes crucial to demonstrate that your product/technology is the “safe” option for investment and a stand-out opportunity by including a clinical roadmap and development strategy of your product/technology. This roadmap illuminates clearly for the investment team what the target indications will be and the paths to […]
The Strategic Clinical Innovation Organization method, developed by BBCR, can help streamline the clinical trial process by operating on the fundamental principle that strategy and design are keys for the success of drug and medical device development.
March 29th, 2022 | SCIOThe SCIO concept is the value proposition solution for cost-effectiveness and risk management in clinical development. Learn more about how BBCR can help.
March 9th, 2022 | SCIOWe are excited about the recognition by the DIGITECH insight magazine that included the BBCR’s proprietary SCIO process within the top 10 innovations in drug development for 2022.
February 9th, 2022 | SCIOBBCR has developed an approach to meet the challenges of researchers and innovators seeking a clearer path to market. Learn more about the advantages of our Strategic Clinical Innovation Organization (SCIO) concept.
December 15th, 2021 | SCIO
We aim to transform the transition stage between pre-clinical and clinical endpoints with smart strategy that, at a minimum, redirects the costs to benefit patients. The FDA has been calling for a smarter, more innovative approach, and we believe SCIO is the integrative, multidisciplinary approach to deliver it. Why SCIO? Investing in Strategy Accelerate Patient […]
Our industry needs innovative strategies, and reduced-risk clinical trials. BBCR clinical development services and drug development consulting integrates real world evidence (RWE) into clinical development plans and regulatory strategies.
November 18th, 2021 | SCIO
Focus must go to cost containment and value-based developments that allow sponsors to move more treatments to market faster. BBCR’s team expertise in rare diseases and precision medicine combined with SCIO approach helps streamline trials for swift regulatory approvals. Real-World Data can be collected and approved by regulators for Evidence Generation Regarding Safety and Effectiveness. […]
Our CRO Management services reduce trial costs while achieving high data quality. BBCR takes a SCIO approach to CRO management that efficiently communicates back to senior management meeting the needs of the new economy and the needs of patients and shareholders.
September 28th, 2021 | SCIO
BBCR takes a SCIO approach to CRO management that efficiently communicates back to senior management meeting the needs of the new economy and the needs of patients and shareholders. BBCR focuses on providing biotech and pharmaceutical companies with an assortment of services tailored to support clinical development programs transitioning from pre-clinical into Phase II PoC […]
The BBCR team of experts works with clients to build a customized strategy in the areas of pre-clinical and clinical product development and regulatory affairs.
June 29th, 2021 | SCIO
We believe that understanding the perspective, challenges, and needs of our clients allows us to serve as a trusted adviser throughout the clinical development process. BBCR clients are domestic and international, small and medium sized drug and device biotechnology firms as well as venture capitalist. Clinical researchers and innovators come to us looking for the […]
Has Covid-19 hampered your clinical research projects? Let BBCR help jump start your product development initiative in order to achieve optimal product market positioning
June 16th, 2021 | SCIO
Given the global Covid19 pandemic and the global nature of the pharmaceutical and biotech industry, key operational areas have and will continue to be impacted. As with any emergency response, the key to management is implementing mitigation plans. Early stage pharmaceutical and biotech companies need to be proactive in developing contingency plans to jump start […]