SCIO Strategic Consulting
SCIO is critical to accelerate approval and significantly reduce costs.
Declining pharmaceutical industry productivity is well recognized by drug developers, regulatory authorities and patient groups. Despite the fast growth of the sector, most of the 1,500 U.S. biotech firms do not make money.
Challenges
One of the major challenges facing this industry is the rising cost of clinical trials. According to a recent FDA report, “The investment required for one successful commercial launch of a medicinal product increased more than 55% in less than a decade, due in large part to the monies required to take a drug from the laboratory and carry it through the standard phases (1-3) of clinical study”.
Thus, it is crucial to ensure that trials are significantly more efficient and cost-effective with the involvement of strategy at the time of IND or IDE submission. It is crucial to ensure that the clinical trial attrition is reduced.
The Future
Big-Picture Thinkers, both within and outside the industry, are involved in the re-examination of the drug development process in an effort to foster fast innovation. MIT’s New Drug Development Paradigms (download here) and Children’s Hospital Informatics Program’s new forecasting models to reduce drug costs are some of the latest developments towards that goal.
With high costs associated with the later phase of development, developing Clinical Proof of Concept and early stage trials are extremely important.
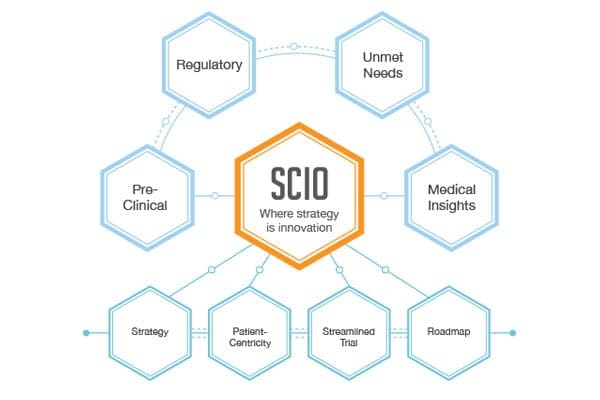
Strategic consulting specialized in early stage development are essential to create study design and reach proof of concept in time frames and reduce costs up to 30%.
BBCR consulting adopts the SCIO process and operates on the fundamental principle that strategy and design are keys for the success of drug and medical device development.
Please help by communicating the need of this overdue change by sharing this post.
We also ask that you consider the following questions and to please share your opinion by providing your comments at the bottom of this page:
- Do you believe that clinical protocol is a crucial step for the success of the trial?
- Do you agree that a thoughtful clinical protocol and trial design would facilitate patient recruitment and data interpretation?

Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.