Given the global Covid19 emergency and the global nature of the pharmaceutical and biotech industry, key operational areas will be impacted.
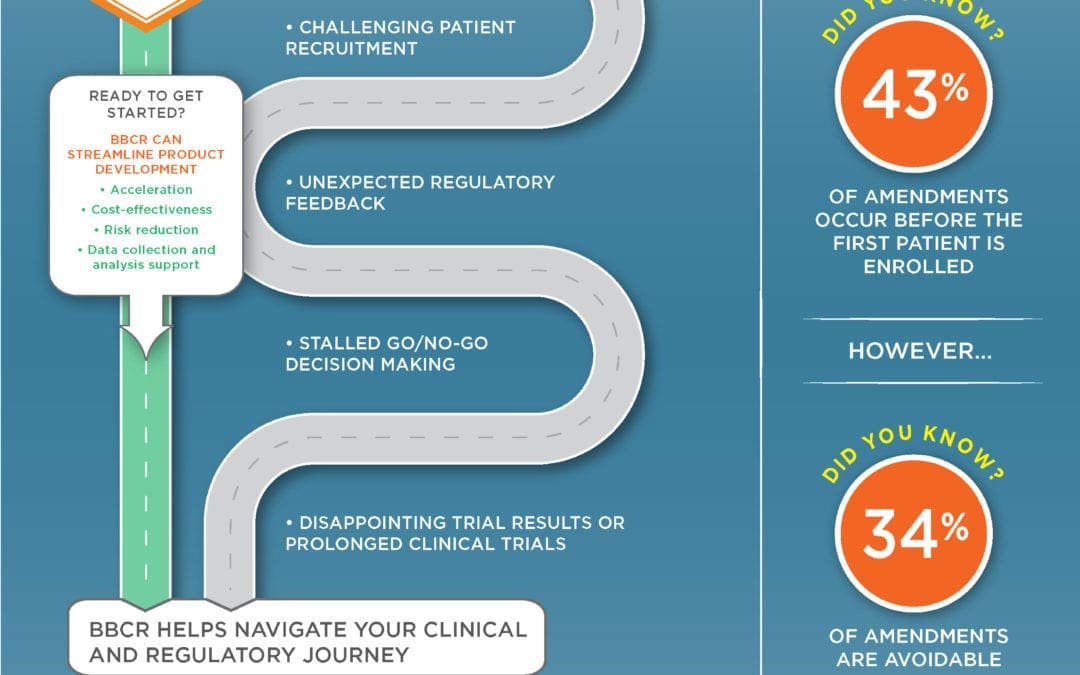
As with any emergency response, the key to management is implementing mitigation plans. Early stage pharmaceutical and biotech companies need to be proactive in developing contingency plans to jump start clinical research after Covid19.
BBCR can help to jump starting your activities and implementing mitigation plans. Click to get started.

Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.