Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process and strategy. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.

Speaking Topic: Increasing Investor’s Evaluation of SME Biotech in a Slumping Market BBCR will be speaking on the challenges investors face and how to successfully navigate today’s Biotech market.

Biologic treatments show promise in providing clinical solutions to a variety of diseases including rare cancers and precision medicine. Related Services Include: Indications analysis and prioritization Strategic drug assessment Clinical study design and protocol Biomarker strategy Early Clinical Development FDA meeting and submission Pre-ND integrated development plan CRO and project management Study remediation and rescue

The BBCR team designs Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies with the drug clinical plan and regulatory strategy in mind. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose for PoC, disease model and biomarkers Investigate drug concentration at the target […]

Our proprietary Strategic Clinical Innovation Organization (SCIO) concept was developed to address and maneuver around evolving challenges, allowing for time and cost efficiencies, and mitigating risk. BBCR works with clients in the areas of Biomarkers, Clinical Research consulting, Regulatory Affairs, and Early Clinical Development. To learn more about how BBCR can help with your research […]

Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. BBCR Consulting offers world-class regulatory, clinical research, and biomarker consulting services that provide high-value, and support our clients’ operational and functional needs. Our process is designed […]

Moderna announced on January 25 plans for testing two different booster vaccines aimed at the SARS-CoV-2 variant B.1.351 that emerged in South Africa and has now spread to numerous countries. Moderna COVID-19 Vaccine produced neutralizing titers against all key emerging variants tested, including B.1.1.7 and B.1.351, first identified in the UK and Republic of South […]

Strategic Clinical Innovation Organization (SCIO) – A Clear Path to Approval The Strategic Clinical Innovation Organization (SCIO) concept developed by BBCR was designed specifically to help pharmaceutical innovators address the concerns and maneuver around evolving challenges. SCIO allows for time and cost efficiencies, and risk mitigation. The BBCR team is armed with extensive clinical, regulatory […]

Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. Click the button below to learn more about how we have helped companies with their clinical trial needs.

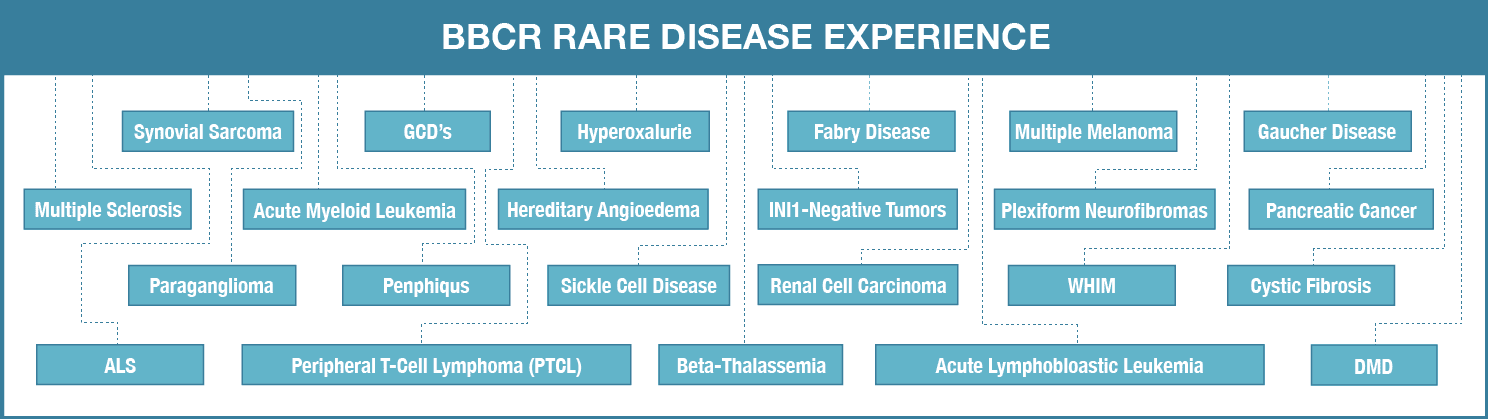
Biotech companies’ continued participation in the Global Orphan Drug Market Opportunity requires the development of new strategies rooted in the established knowledge of orphan developer experts. Participation of big pharmaceutical companies in the ongoing clinical research of orphan drug are about to introduce a drastic change in overall scenario of the approach. Global Orphan Drug […]