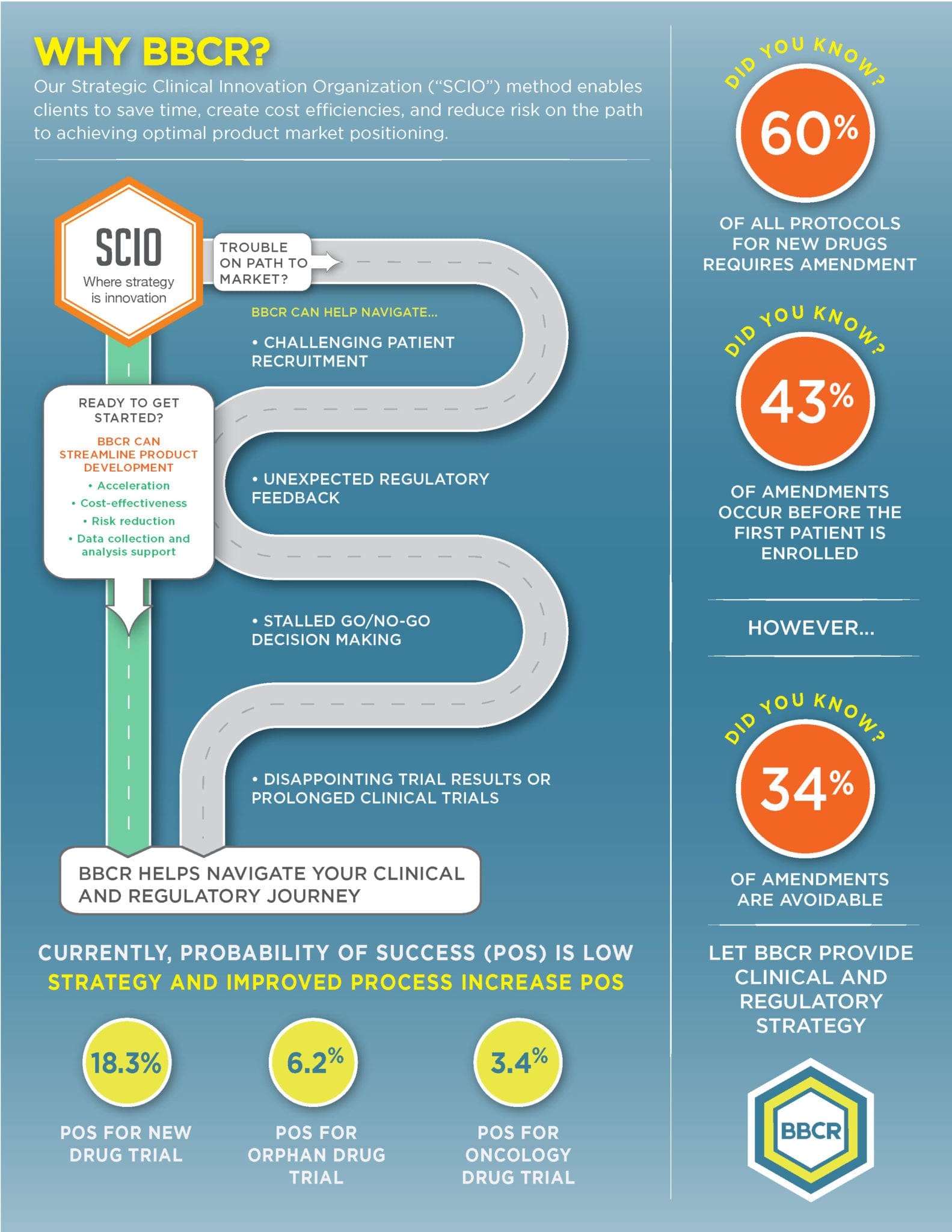
BBCR specializes in the strategy and delivery of early-phase clinical development services to enable informed, timely decision making for our clients. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose for PoC, disease model and biomarkers Investigate drug concentration at the target site of action Investigate […]

BBCR specializes in the strategy and delivery of early-phase clinical development services to enable informed, timely decision making for our clients. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose for PoC, disease model and biomarkers Investigate drug concentration at the target site of action Investigate […]

According to recently released data, 12 out of the 28 drugs approved in the first quarter of 2020 by the FDA were repurposed drugs. The drug repurposing approval process is an effective tool that accelerates the development of drugs for new indications or drug reformulation. In addition, the development of drug repurposing compared to a […]

Drug repurposing acts to lower the need for early stage clinical trials and can help identify new uses for existing drugs. People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. One attractive option of Drug Repurposing is to use a scientific approach […]

Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. BBCR Consulting offers world-class regulatory, clinical research, and biomarker consulting services that provide high-value, and support our clients’ operational and functional needs. Our process is designed […]

Given the global Covid19 emergency and the global nature of the pharmaceutical and biotech industry, key operational areas will be impacted. As with any emergency response, the key to management is implementing mitigation plans. Early stage pharmaceutical and biotech companies need to be proactive in developing contingency plans to jump start clinical research after Covid19. […]