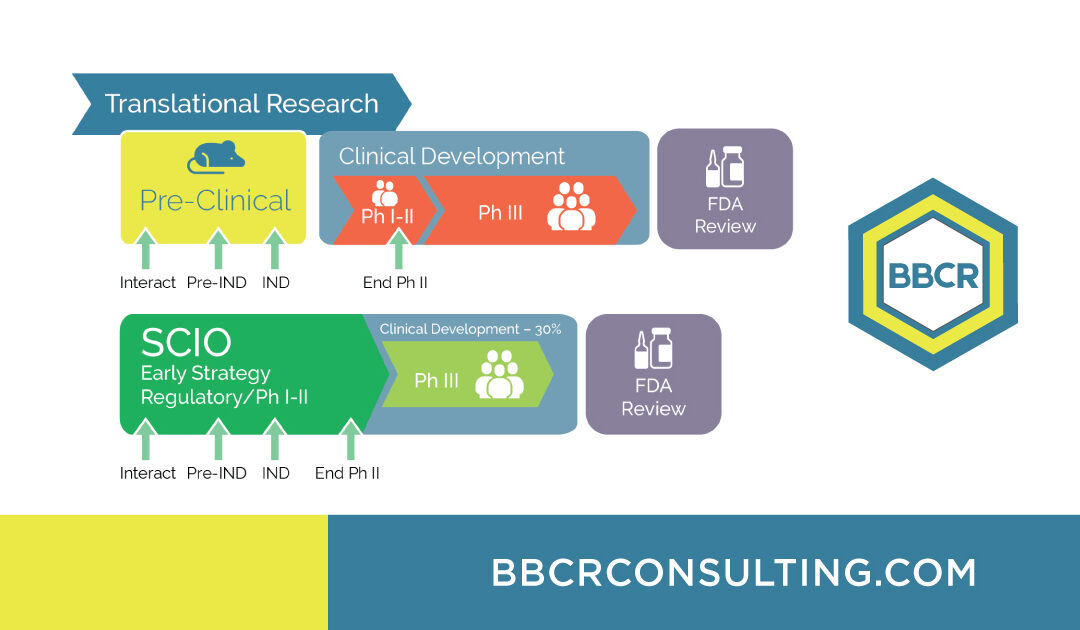
With BBCR’s Strategic Clinical Innovation Organization (SCIO℠) method, we’re redefining how innovation meets efficiency in drug development—providing a clear, data-driven path to market approval.
The SCIO℠ method works by learning and predicting—analyzing each pre-clinical asset to identify its true potential and forecasting the level of certainty for every strategic option. This proactive approach empowers smarter decisions early in development, leading to stronger, faster outcomes.
In today’s world of complex molecular innovation, yesterday’s assumptions can’t deliver tomorrow’s breakthroughs. Data from marketed products show that even identical molecules can yield vastly different results depending on the strategy behind them. Early evaluation, indication selection, and validation through Phase I-II trials create a competitive edge that late-stage pivots simply can’t match.
With our SCIO℠ method, BBCR helps you shape smarter strategies, reduce uncertainty, and unlock the full potential of your drug opportunity.

Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.