SCIO Strategic Consulting
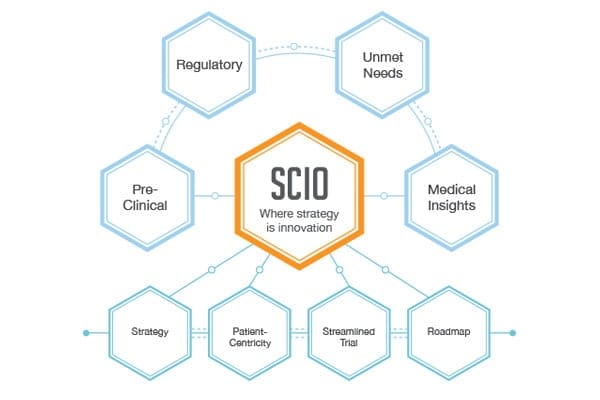
The creation of the concept for a Strategic Clinical Innovation Organization (SCIO) has been evolving over the past 10 years as the market has become aware of the risk from the lack of “upfront” clinical strategy and development planning. This process has been given added momentum with the advent of discovery drugs with potential multiple indications and the new class of device/drug combination therapeutics.
For more than 20 years, data-monitoring has been supporting biotechnology companies during the fundraising as well as licensing stages. In recent years, venture capital funds have become much more hesitant to invest in the biotechnology sector so it is crucial to make as strong a business case as possible to ensure that you secure the available funding as opposed to other opportunities. One of the key issues is making sure venture capital funds do not undervalue your product/technology proposition to potential investors. To enhance your position, it becomes crucial to demonstrate that your product/technology is the “safe” option for investment and a stand-out opportunity by including a clinical roadmap and development strategy of your product/technology. This roadmap illuminates clearly for the investment team what the target indications will be and the paths to achieving a first cycle success.
What has become more important, and is somewhat new to the process, is the higher hurdles being erected by Regulators on the clinical study design and expected endpoint evaluation. This shift is clearly an effort to rebalance the process by focusing the clinical development component on science. This means we move from doing trials and taking the end data to make our case for release to one of setting up each trial in a concrete but scientifically transparent manner and letting the data fall as it may. The latter requires a different method of reducing the “gap” between the discovery phase and the protocol implementation. Thereby reducing the risk inherent in the traditional method. It is essential to have a scientific and thorough clinical development plan that encompasses all the strategies and decision nodes needed for execution. SCIO’s, recognizing the missed segment of the chain, fill the gap by providing customized thinking and clinical planning that reduce the risk while accelerating the scientific underpinning of the program.
Investors want to know how much their ROI will be and by showing them that you have also put together an equally strong business proposition alongside SCIO scientific roadmap and strategic clinical plan will go a long way in differentiating yourself from the competitors seeking the same funding.

Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.