Nasopharyngeal swabs are considered the gold standard to accurate test COVID19 infection.
Unfortunately, these tests require specific supplies, some significant time for results, and place health care workers at risk of infection. Thus, clinicians and researchers have been looking for alternatives to nasopharyngeal swabs since the early days of the pandemic.
Recently, it has been demonstrated the saliva’s role in testing for COVID-19.
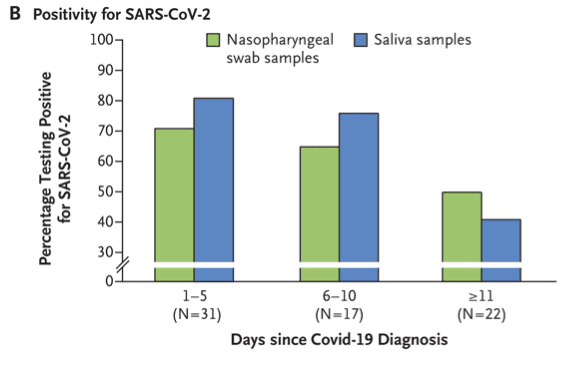
A recently commentary in the New England Journal of Medicine (https://www.nejm.org/doi/full/10.1056/NEJMc2016359) reported the findings on an a head-to-head comparison of saliva or nasopharyngeal swab specimens for detection of sars-cov-2.
Rigorous evaluation was performed to determine how saliva specimens compare with nasopharyngeal swab specimens with respect to sensitivity in detection of SARS-CoV-2 during the course of infection.
Among 70 patients tested with suspected cases of COVID-19 in a controlled health care setting, saliva samples often contained more copies of SARS-CoV-2 than did swab samples, and a higher percentage of saliva samples were positive.
When the saliva testing was adopted in a mass screening in a rural area (https://www.medrxiv.org/content/10.1101/2020.09.23.20150961v1) is showed to be was less sensitive than nasopharyngeal swabs. Indeed, when the viral load was low the two methods disagreed more often. That’s because the virus is more difficult to detect using either method in people who have very recently become infected or those tested on the tail end of their illness. Among asymptomatic patients, saliva’s sensitivity was only 24 percent compared to swabs.
In conclusion, the saliva testing for COVID19 detection is finally available to clinicians as a valid alternative to nasopharyngeal swabs.

Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.