Multiple SARS-CoV-2 variants are circulating globally. Several new variants have emerged in the fall of 2020. Many variants are relatively unremarkable. But scientists have been keeping a close watch on three rapidly spreading variants—first identified in the UK, South Africa, and Brazil—which harbor an unusual constellation of mutations. They all share a mutation that affects […]

Moderna announced on January 25 plans for testing two different booster vaccines aimed at the SARS-CoV-2 variant B.1.351 that emerged in South Africa and has now spread to numerous countries. Moderna COVID-19 Vaccine produced neutralizing titers against all key emerging variants tested, including B.1.1.7 and B.1.351, first identified in the UK and Republic of South […]

FDA guidance (March 2020 and updated July 2020) acknowledged that the impact of COVID-19 may require companies conducting clinical trials to consider virtual patient visits or put new processes in place regarding their current protocols. Since COVID 19 has changed many of our normal way to conduct Clinical research, most organizations reported some level of […]

The publication suggesting a link between COVID-19 and Magnetism has been retracted. The study, published in a peer-reviewed journal, has attracted widespread derision from researchers. The study titled “Can Traditional Chinese Medicine provide insights into controlling the COVID-19 pandemic: Serpentinization-induced lithospheric long-wavelength magnetic anomalies in Proterozoic bedrocks in a weakened geomagnetic field mediate the aberrant […]

Early Phase III data from Pfizer and BioNTech BNT162b2 is the experimental mRNA-based vaccine candidate from Pfizer and BioNTech’s in Phase III study. Since June, 43,538 participants have been enrolled to date, 38,955 of whom have received a second dose of the vaccine candidate as of November 8. On Monday, November 9th, the company announced […]

Observational data suggest that SARS-CoV-2 spreads between people at short distances. SARS-CoV-2 may spread by the primary method of transmission of the SARS-CoV-2 virus—via relatively large respiratory droplets, which fall quickly to the floor under their own weight, or even via the smaller aerosolized droplets capable of circulating in the air for long periods of […]

Nasopharyngeal swabs are considered the gold standard to accurate test COVID19 infection. Unfortunately, these tests require specific supplies, some significant time for results, and place health care workers at risk of infection. Thus, clinicians and researchers have been looking for alternatives to nasopharyngeal swabs since the early days of the pandemic. Recently, it has been […]

Pfizer, a pharmaceutical company, in partnership with BioNTech has reported that its lead vaccine candidates has shown promising results in protecting monkeys against coronavirus infections. Pfizer ‘s experimental vaccine is different from other vaccine candidates. This is in an mRNA format, which should allow for a better migration of the viral gene into the cell […]

Blood-based immunological changes that are linked to the disease and, in some cases, to symptom severity were published in Nature Medicine Source: (https://www.nature.com/articles/s41591-020-1038-6). For a disease with such diversity of symptoms and outcomes, and in patients of different ages and sexes with different underlying conditions, finding a common immune pattern of the immune response to […]

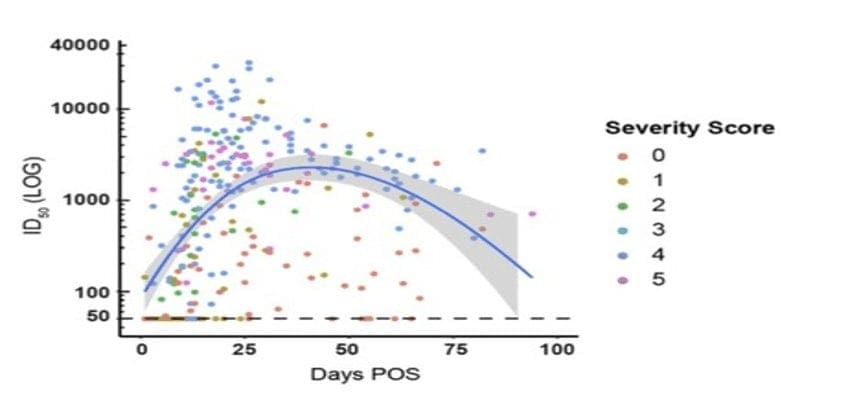
A transient neutralizing Ab (nAb) response is a feature shared by both COVID19 infection and the coronaviruses that cause common colds (https://www.medrxiv.org/content/10.1101/2020.07.09.20148429v1) Antibody (Ab) responses to SARS-CoV-2 can be detected in most infected individuals 10-15 days following the onset of COVID-19 symptoms. Seroconversion occurred in >95% of cases and neutralizing antibody (nAb) responses. All subjects, […]