
Streamlining Clinical Development with Clinical and Regulatory Expertise Our team will empower you with medical insight, practical regulatory, translational medicine, and clinical research expertise, and streamlined clinical trials.

Streamlining Clinical Development with Clinical and Regulatory Expertise Our team will empower you with medical insight, practical regulatory, translational medicine, and clinical research expertise, and streamlined clinical trials.

Clinical trial results have been promising, and the next generation of Cell and Gene therapies holds tremendous promise for many patients and families. The development of Cell and Gene Therapies is challenging due to complex study designs, adequately monitoring of adverse reactions, long-term immune response and safety follow-up. BBCR has successfully delivered cost-effective clinical plans […]
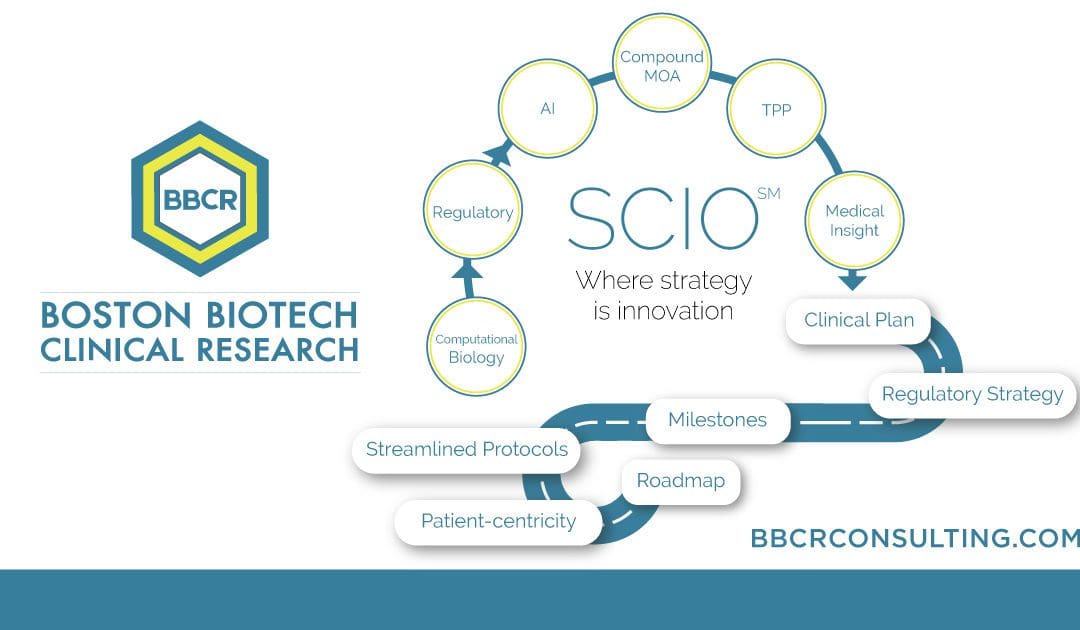
The Strategic Clinical Innovation Organization SCIO SM (SCIO) method is explicitly designed to help pharmaceutical innovators address their concerns and maneuver around evolving challenges. SCIO SM allows for time and cost efficiencies and relief of risk management. The SCIO SM method aims to learn, predict and make better decisions for a successful drug opportunity. This […]
The Trial Management That Addresses Risks Experienced management address risks such as: Delayed enrollment Multiple protocol amendments Poor patient retention Poor data quality Prolonged trial completion timeline Poor safety monitor Let BBCR Consulting assist in your clinical trial strategy. Reach out to us to learn more about how we can help.
How can SCIO SM help? To enhance your position, it becomes crucial to demonstrate that your product/technology is the “safe” option for investment and a stand-out opportunity by including a clinical roadmap and development strategy of your product/technology. This roadmap illuminates clearly for the investment team what the target indications will be and the paths […]
Developing drugs with substantial benefits in smaller, molecularly defined, pharmacologically relevant subpopulations of patients with the same clinically recognized disease is increasingly being viewed as a viable pathway for bringing drugs to market.
Developing innovative or repurposing drugs for orphan diseases can be rewarding, but navigating the challenges is not for the faint of heart. Expert guidance is essential in an area where patients are few; a lack of previous studies may hamper progress as you mount an orphan petition and negotiate a clinical plan with the FDA. […]

As one of the first consultancy teams to streamline clinical trials, BBCR’s boutique consulting team specializes in rare disease and orphan indications dedicated to supporting pharmaceutical innovators, and nurturing each product’s strengths. The BBCR Team provides knowledge in cell, biologics and gene therapies BBCR addresses sponsors’ questions in the ever-changing regulatory environment Clinical strategy for […]

People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. About a third of orphan approvals by the FDA since the program began have been mostly for repurposed mass-market drugs. BBCR’s team of industry experts can help match treatments to rare genetic conditions […]

BBCR helps orphan drug developers find direction in clinical trials involving biologics, biosimilars, small molecules, medical devices, and repurposing. BBCR consultants have the experience to guide you through the development process with a clinical plan and a regulatory strategy. Our team will empower you with medical insight, effective regulatory expertise, strategy, and streamlined early clinical […]