The Trial Management That Addresses Risks Experienced management address risks such as: Delayed enrollment Multiple protocol amendments Poor patient retention Poor data quality Prolonged trial completion timeline Poor safety monitor Let BBCR Consulting assist in your clinical trial strategy. Reach out to us to learn more about how we can help.
Tagged: SCIO
Clinical trials are time-consuming, expensive, and often burdensome on patients. Clinical trials can fail for many reasons. Our experience provides an understanding of these reasons and offers insights into opportunities for improving the likelihood of creating and executing successful clinical trials.
November 16th, 2022 | SCIOSCIO assists with early stage biotech company evaluation. Learn why adopting this clinical roadmap and development strategy is critical to your success in bringing your product to market.
October 28th, 2022 | SCIOHow can SCIO SM help? To enhance your position, it becomes crucial to demonstrate that your product/technology is the “safe” option for investment and a stand-out opportunity by including a clinical roadmap and development strategy of your product/technology. This roadmap illuminates clearly for the investment team what the target indications will be and the paths […]
An attractive option of drug repurposing is to use a scientific approach to identify new uses for existing drugs thereby reducing the need for early stage clinical trials.
August 17th, 2022 | SCIO
People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. About a third of orphan approvals by the FDA since the program began have been mostly for repurposed mass-market drugs. BBCR’s team of industry experts can help match treatments to rare genetic conditions […]
Strategic Consulting and the SCIO Process – Paving the Way for Drug Development
July 19th, 2022 | SCIOSCIO is critical for accelerating approval and significantly reducing the costs of drug and medical device development. One of the major challenges facing this industry is the rising cost of clinical trials. Thus, it is crucial to ensure that trials are significantly more efficient and cost-effective with the involvement of strategy at the time of […]
SCIO helps early stage biotech company evaluation. Learn why adopting this clinical roadmap and development strategy is critical to your success in bringing your product to market.
April 20th, 2022 | SCIO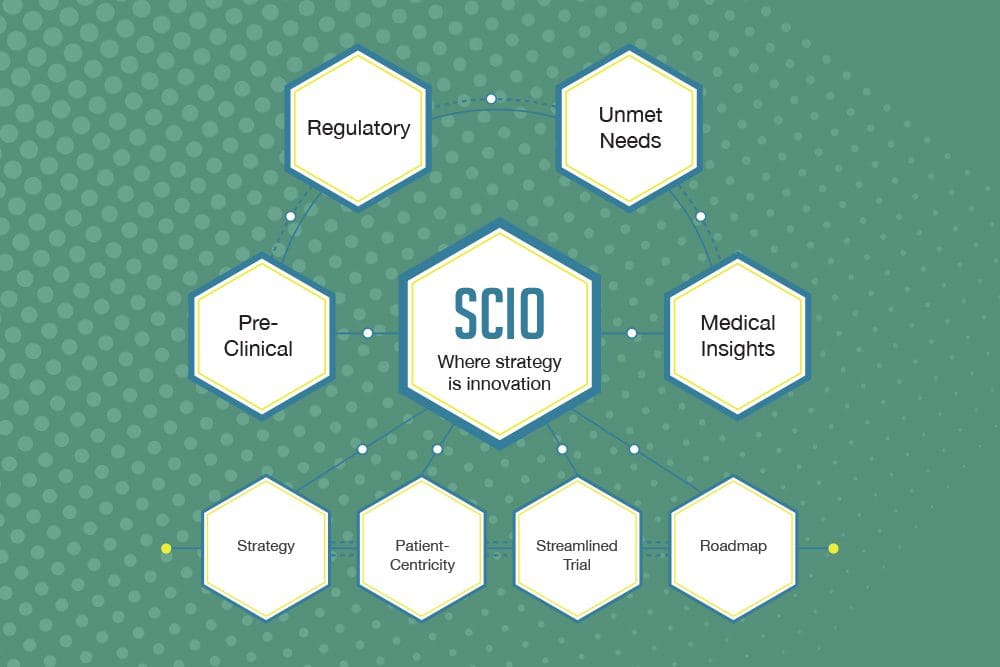
How can SCIO help? To enhance your position, it becomes crucial to demonstrate that your product/technology is the “safe” option for investment and a stand-out opportunity by including a clinical roadmap and development strategy of your product/technology. This roadmap illuminates clearly for the investment team what the target indications will be and the paths to […]
The Strategic Clinical Innovation Organization method, developed by BBCR, can help streamline the clinical trial process by operating on the fundamental principle that strategy and design are keys for the success of drug and medical device development.
March 29th, 2022 | SCIOThe SCIO concept is the value proposition solution for cost-effectiveness and risk management in clinical development. Learn more about how BBCR can help.
March 9th, 2022 | SCIOWe are excited about the recognition by the DIGITECH insight magazine that included the BBCR’s proprietary SCIO process within the top 10 innovations in drug development for 2022.
February 9th, 2022 | SCIOBBCR has developed an approach to meet the challenges of researchers and innovators seeking a clearer path to market. Learn more about the advantages of our Strategic Clinical Innovation Organization (SCIO) concept.
December 15th, 2021 | SCIO
We aim to transform the transition stage between pre-clinical and clinical endpoints with smart strategy that, at a minimum, redirects the costs to benefit patients. The FDA has been calling for a smarter, more innovative approach, and we believe SCIO is the integrative, multidisciplinary approach to deliver it. Why SCIO? Investing in Strategy Accelerate Patient […]
Our industry needs innovative strategies, and reduced-risk clinical trials. BBCR clinical development services and drug development consulting integrates real world evidence (RWE) into clinical development plans and regulatory strategies.
November 18th, 2021 | SCIO
Focus must go to cost containment and value-based developments that allow sponsors to move more treatments to market faster. BBCR’s team expertise in rare diseases and precision medicine combined with SCIO approach helps streamline trials for swift regulatory approvals. Real-World Data can be collected and approved by regulators for Evidence Generation Regarding Safety and Effectiveness. […]