BBCR Voice
From the Perspective of Researchers, Clinicians, and Regulatory Experts
The most efficient path in the clinical research process is a moving target. Technology innovation and regulatory requirements require constant updates. Through BBCR Voice, we aim to share not only our knowledge and expertise but also solutions to current challenges. BBCR embraces the challenges of developers and investors seeking a more straightforward path to market.
Recent Posts
Has Covid-19 hampered your clinical research projects? Let BBCR help jump start your product development initiative in order to achieve optimal product market positioning
Given the global Covid19 pandemic and the global nature of the pharmaceutical and biotech industry, key operational areas have and will continue to be impacted. As with any emergency response, the key to management is implementing mitigation plans. Early stage...
The Strategic Clinical Innovation Organization (SCIO) concept developed by BBCR was designed specifically to help pharmaceutical innovators address the concerns and maneuver around evolving challenges. SCIO ensures time and cost efficiencies, and risk mitigation.
BBCR has developed an approach to meet the challenges of researchers and innovators seeking a clearer path to market. Flexible Road-Map Successful clinical development, for drug and medical device companies (small and large), requires an early strategy with a flexible...
BBCR’s team of industry experts help our clients match treatments to rare genetic conditions and unsolved diseases, then work with a product developer on the best plan to market.
Drug repurposing acts to lower the need for early stage clinical trials and can help identify new uses for existing drugs. People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. One...
Boston Biotech Clinical Research uses innovative approaches to de-risk your product development. For our clients interested in Proof of Mechanism and Proof of Concept – PoM and PoC – BBCR has the expertise to ensure successful product development at any stage of development.
The BBCR team designs Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies with the drug clinical plan and regulatory strategy in mind. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose...
Application of CAR Cell Therapy in Solid Cancer
From CAR T to CAR macrophage: The improvement of CAR cell therapy in solid cancer treatment The Chimeric Antigen Receptor T (CAR T) cell technology is a revolutionary therapy and has shown promising clinical response in cancer treatment. In 2017, anti-CD19 CAR T cell...
Electrographic Seizures and COVID-19
Electrographic seizures and other epileptiform patterns are common in patients with COVID-19 and associated with adverse outcomes By: Dr. Maria Niu There have been 137,866,311 confirmed cases of COVID-19, including 2,965,707 deaths in the world, according to the WHO...
Obesity Associated Gut Microbiota Alterations Worsen GVHD
Obesity-induced gut microbiome composition is linked with graft-versus-host disease (GVHD) in mice and human after allogeneic hematopoietic stem cell transplantation (HSCT) By: Dr. Maria Niu GVHD is a potentially severe immune disorder related to HSCT. In brief, the...
Recent Advance of Gene Therapy in Rare Diseases
Gene Therapy improved vision in patients with leber hereditary optic neuropathy (LHON) By: Dr. Maria Niu Mitochondria, powerhouses of eukaryotic cells, is a certain kind of cytoplasmic organelle and plays a critical role in energy production. Mitochondria dysfunction...
New Drug Approved in the Field of SiRNA-based Therapeutics Targeting Rare Disease
Lumasiran, a siRNA Therapeutic drug for primary hyperoxaluria type1(PH1) By: Dr. Maria Niu PH1 is a rare genetic disease caused by deficiency of the liver peroxisomal enzyme alanine:glyoxylate-aminotransferase (AGT), resulting in overproduction of oxalate. The...
COVID-19 Pandemic and Mental Health
The Lancet: 34% COVID-19 survivors have neurological and psychiatric disorders By: Dr. Maria Niu Previous case reports had described the altered mental problems and psychiatric disorders in people diagnosed with COVID-19 infection. In the latest study...
The Effect of Fecal Transplant on Melanoma
Fecal microbiota transplantation (FMT) promotes the response of check point inhibitors (CPI) to melanoma patients By: Dr. Maria Niu Fecal microbiota transplantation (FMT) is a procedure that delivers fecal bacteria and other microbes from a healthy individual into...
Drug Development for Rare Duchenne Muscular Dystrophy (DMD)
Progress and limitation of therapeutic approaches targeting DMD By: Dr. Maria Niu Duchenne muscular dystrophy (DMD) is an X—a chromosome-linked recessive disorder caused by mutations in the gene coding muscle cytoskeletal protein dystrophin. Because of the X-linked...
Mutants: the challenges of COVID 19 Vaccine
True or false: Virus variants weaken the effectiveness of COVID 19 Vaccine Coronavirus vaccines have significantly reduced the infectiousness of COVID 19 cases and showed significant protective efficacy against COVID 19. However, the recent reported COVID19 variants...
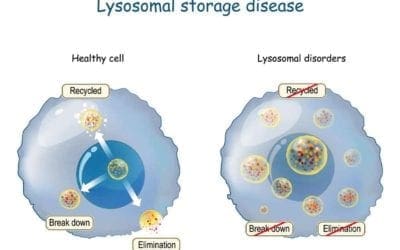
Cell and gene advanced therapy in the Battle for Lysosomal Storage Disease
Emerging cell and gene therapy may offer sustained long-term correction for LSD patients Dr. Maria Niu Lysosomal storage diseases (LSDs) are rare inherited metabolic diseases and characterized by the accumulation of substrates in excess in various organs' cells due to...
BBCR offers innovative approaches to de-risk your product development during Early Clinical Development
Depending on your project goals, our consultants work with you to design cost-effective early clinical studies that hold potential for reducing Phase III failures. Our services include: POM & POC Translational Research Clinical Plan & Study Design Phase 1...
BBCR has experience in biologics for rare diseases and can assist with the development of a targeted strategy to meet your study needs.
Biologic treatments show promise in providing clinical solutions to a variety of diseases including rare cancers and precision medicine. Services include: Indications analysis and prioritization Strategic drug assessment Clinical study design and protocol Biomarker...
Our Strategic Clinical Innovation Organization or SCIO method enables our clients to save time, create cost efficiencies, and reduce risk on the path to achieving optimal product market positioning. Reach out to BBCR today to learn more.
Our Strategic Clinical Innovation Organization (“SCIO”) method enables our clients to save time, create cost efficiencies, and reduce risk on the path to achieving optimal product market positioning. The Strategic Clinical Innovation Organization (SCIO) concept...
Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to help streamline the clinical trial process.
Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. BBCR Consulting offers world-class regulatory, clinical research,...
Depending on your project goals, BBCR can work with you to design cost-effective early clinical studies that hold potential for reducing Phase III failures.
Expertise in integration of in vitro and in vivo analysis during early clinical research is a critical development milestone for efficient candidate development. In addition, the BBCR team guides in identifying the right target, the right biomarker, the right safety,...
In rare diseases and precision medicine, implementation of biomarkers during product translation into clinic and early clinical development moves treatment to market faster. BBCR is experienced in helping you understand the role of biomarkers and how to move your product to market more efficiently.
Biomarkers are considered a routine part of drug development. Learn more about how BBCR can help get your product to market more efficiently with proper plan development and strategy. Plan Development Insightful strategy is the most effective way to ensure quality...
Covid-19 vaccine reduces infectiousness—a key factor in slowing virus spread.
Preliminary results from an Israel-based study suggest that one dose of the Pfizer’s vaccine reduces viral load- a key factor in slowing virus spread. Several of the approved to market COVID-19 vaccines are over 90% effective in preventing disease. But no much data...
Identification and adoption of biomarkers are especially valuable in rare disease and precision medicine product development. Learn more about how BBCR can help.
Biomarkers are now a routine part of drug development The FDA recognizes biomarker development as a high priority area for future research. The FDA and European Medicines Agency (EMA) have developed similar processes for the qualification of biomarkers intended for...
New Spike Mutants from SARS-CoV-2
Multiple SARS-CoV-2 variants are circulating globally. Several new variants have emerged in the fall of 2020. [button...
Moderna COVID-19 Vaccine Protects Against New COVID Variants
Moderna announced on January 25 plans for testing two different booster vaccines aimed at the SARS-CoV-2 variant B.1.351 that emerged in South Africa and has now spread to numerous countries. Moderna COVID-19 Vaccine produced neutralizing titers against all key...
COVID-19 Severity and Immune Response Tied to Gut Bacteria
Scientists report that gut microorganisms in COVID-19 patients were very different from those in uninfected individuals and lack of good bacteria that regulate our immune system. The presence of an abnormal gut bacteria persists after the virus is gone and may be...