BBCR Voice
From the Perspective of Researchers, Clinicians, and Regulatory Experts
The most efficient path in the clinical research process is a moving target. Technology innovation and regulatory requirements require constant updates. Through BBCR Voice, we aim to share not only our knowledge and expertise but also solutions to current challenges. BBCR embraces the challenges of developers and investors seeking a more straightforward path to market.
Recent Posts
BBCR specializes in strategy and provides early clinical research services to enable informed, timely decision-making for our clients. It is our mission to support domestic and international pharma, biotech, and device companies by nurturing their products’ strengths while improving efficiency and safety.
Developing innovative or repurposed drugs for orphan diseases can be rewarding, but navigating the challenges is not for the faint of heart. Our regulatory and clinical research consultants are experts in biologics, cell and gene therapies, and small molecule...
Clients entrust BBCR to provide expert guidance in simplifying their clinical plans and developing customized strategies and cost-effective clinical trials that nurture product strengths.
Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. Click the button below to learn more about how we have helped...
BBCR has been invited to speak at the upcoming 12th Clinical Trials Strategic Summit 2023 May 10th- 11th 2023 in Boston, MA
Speaking Topic: Increasing Investor’s Evaluation of SME Biotech in a Slumping Market BBCR will be speaking on the challenges investors face and how to successfully navigate today's Biotech market.
Welcome to BBCR’s next generation website! We invite you to come explore our new site, redesigned to provide a clear overview of how BBCR can help you with early clinical development, rare disease and orphan drug solutions.
We are pleased to share with you our newly enhanced website. Over the past several months, our team has carefully reviewed our processes, capabilities, and offerings in order to provide you with the clearest overview of how we help our clients. BBCR is dedicated to...
Translational Medicine and Epigenetics Program Consultation bridges the gap between pre-clinical and early clinical development. We invite you to learn more about how BBCR can help with your clinical development needs.
Translational medicine is a bridge between pre-clinical and early clinical development (from bench to bedside), creating the foundation for precision medicine in late clinical development. A translational medicine program includes the adopting biomarkers to mitigate...
Drug repurposing offers your product shorter journeys to the clinics. It is especially crucial to rare and neglected diseases, cancers, and neurodegenerative diseases. Learn how we can help at bbcrconsulting.com
The BBCR consultants, experienced in orphan, rare and ultra-rare diseases, have advised biotech and venture capitalists for new indications evaluation and prioritization. BBCR’s team of industry experts can help match treatments to rare genetic conditions and unsolved...
BBCR provides expert guidance for Orphan drug development. Our consultants have the experience to guide you through the Orphan clinical research process with a clinical plan and regulatory strategy for an accelerated approval.
Streamlining Clinical Development with Clinical and Regulatory Expertise Our team will empower you with medical insight, practical regulatory, translational medicine, and clinical research expertise, and streamlined clinical trials. [button...
BBCR maintains a philosophy of fostering continual improvement and productivity in the areas of Medical Affairs and Clinical Research
Our goal is to optimize every aspect of the research and development process We will: Improve protocol development, including current trends in clinical trial designs and endpoints Benchmark relevant historical trial enrolment with unmet needs and ongoing trial...
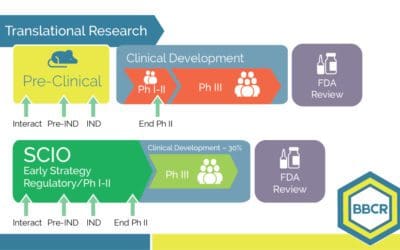
Build the first step for a successful study – An early strategy ensures efficiency in Clinical Development
Designed primarily to meet regulatory requirements for therapies targeting large patient populations, the existing development model lacks flexibility and efficiency to address today’s drug development demands. Challenges include managing product pipelines, targeting...
Cell and Gene therapy are the new frontiers in the fight against devastating diseases, including rare diseases and cancers.
Clinical trial results have been promising, and the next generation of Cell and Gene therapies holds tremendous promise for many patients and families. The development of Cell and Gene Therapies is challenging due to complex study designs, adequately monitoring of...
Due Diligence is critical before dedicating precious time and resources to a research project that appears to hold commercial promise.
Due Diligence is typically performed by investors but can, and should, be conducted by sponsors to increase their chance of financing success, partnering, mergers, acquisitions (M&As), and product licensing. For companies developing multiple products, Due...
BBCR’s SCIO method aims to learn, predict and make better decisions for a successful drug opportunity.
Learn the potentials of each preclinical asset, and predict the level of certainty for each strategic option. Today’s technological molecules require innovation in the development process. We cannot keep using yesterday’s assumptions for tomorrow’s drugs. Marketed...
A translational medicine program includes the adoption of biomarkers to mitigate Phase I and II clinical risks, identify patient subpopulations, facilitate decision-making, and accelerate the release of a drug on the market.
BBCR’s epigenetic expert, Dr. Claudio Carini, has been a pioneer in epigenetics leading the effort of major pharmaceutical companies. BBCR helps to bridge the gap between pre-clinical and early clinical development. Translational medicine is a bridge between...
BBCR’s regulatory and clinical research consultants are experts in biologics, cell and gene therapies, and small molecule developments. Identifying areas of need or economic interest can help sponsors find homes for treatments for orphan indications.
Expert guidance is essential in an area where patients are few; a lack of previous studies may hamper progress as you mount an orphan petition and negotiate a clinical plan with the FDA. BBCR consultants have the experience to guide you through the Orphan clinical...
Clients entrust BBCR to provide expert guidance in simplifying clinical plans and developing customized strategies and cost-effective clinical trials that nurture product strengths.
Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. Click the button below to learn more about how we have helped...
RWE can accelerate approval timelines and reduce drug development costs as long as sponsor companies carefully uphold established evidence and engage in early dialogue with the FDA.
Real-world evidence (RWE) is increasingly important in clinical trial design and drug approval. RWE is clinical evidence related to patient health status and treatment safety and efficacy that are generated in clinical practice. BBCR's team understands the role RWE...
Cell and Gene therapy are the new frontiers in the fight against devastating diseases, including rare diseases and cancers. Clinical trial results have been promising, and the next generation of Cell and Gene therapies holds tremendous promise for many patients and families.
Early-Clinical Research for Cell and Gene Therapy The development of Cell and Gene Therapies is challenging due to complex study designs, adequately monitoring of adverse reactions, long-term immune response and safety follow-up. BBCR has delivered clinical plans and...
Interest is increasing rapidly in using surrogate markers as primary measures of the effectiveness of investigational drugs in definitive drug trials.
The primary difference between a biomarker and a surrogate marker is that a biomarker is a “candidate” surrogate marker. In contrast, a surrogate marker is a test used and taken to measure the effects of a specific treatment. While the current law and regulations...
Due Diligence is critical before dedicating precious time and resources to a research project that appears to hold commercial promise.
Increase investors’ evaluation in a competitive market. Due Diligence is typically performed by investors but can, and should, be conducted by sponsors to increase their chance of financing success, partnering, mergers, acquisitions (M&As), and product licensing....
BBCR provides expert strategies for Orphan Disease. The BBCR mission is to customize development plans, simplify clinical research, design cost-effective trials, streamline protocols, and create a regulatory roadmap.
As one of the first consultancy teams to streamline clinical trials, BBCR’s boutique consulting team specializes in rare disease and orphan indications dedicated to supporting pharmaceutical innovators, and nurturing each product’s strengths. The BBCR Team provides...
A recent posting by Value Market Research highlighted BBCR Consulting among others relating to the global oncology Biomarker market and its projected broad growth.
BBCR=Simplifying Clinical Research Interest is increasing rapidly in using surrogate markers as primary measures of the effectiveness of investigational drugs in definitive drug trials. The primary difference between a biomarker and a surrogate marker is that a...
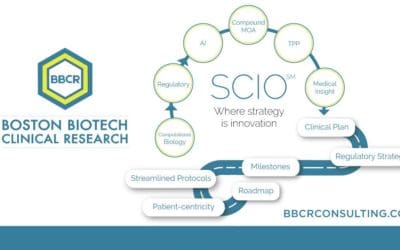
With BBCR’s Strategic Clinical Innovation Organization (SCIO) method, we are able to provide a clear path to market approval.
The Strategic Clinical Innovation Organization SCIO SM (SCIO) method is explicitly designed to help pharmaceutical innovators address their concerns and maneuver around evolving challenges. SCIO SM allows for time and cost efficiencies and relief of risk management....
The BBCR mission is to develop customized strategies, simplify clinical plans and cost-effective trials, streamlined protocols, and create regulatory roadmaps. We invite you to learn more at bbcrconsulting.com.
BBCR’s boutique consulting team specializes in rare diseases and orphan indications and dedicated to supporting pharmaceutical innovators, and nurturing each product’s strengths. The BBCR team provides consultancy in cell, biologics, and gene therapies BBCR addresses...
BBCR’s experienced team in Regulatory Affairs FDA and EMA assists clients in simplifying complex and evolving regulatory processes.
BBCR specializes in taking the complexity inherent to the regulatory phase of the approvals and simplifying it by strategizing our support to each client based on product-specific regulatory requirements and functional needs. Our expertise spans: Regulatory Strategy...
BBCR uses innovative approaches to de-risk your product development. For our clients interested in Proof of Mechanism and Proof of Concept – PoM and PoC – Our team has the expertise to ensure successful product development at any stage of development.
The BBCR team designs Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies with the drug clinical plan and regulatory strategy in mind. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose...