Find opportunity for efficiencies early in the clinical development process
A Clear Path to Approval
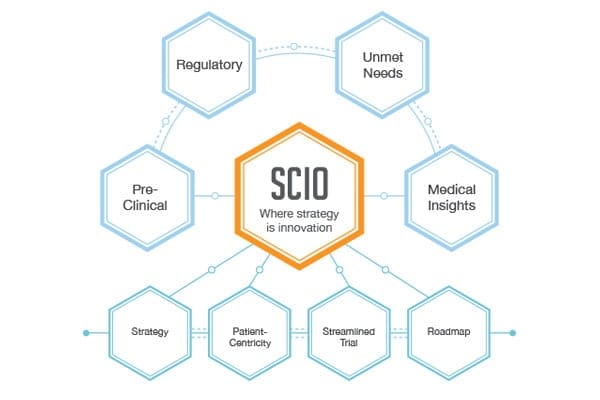
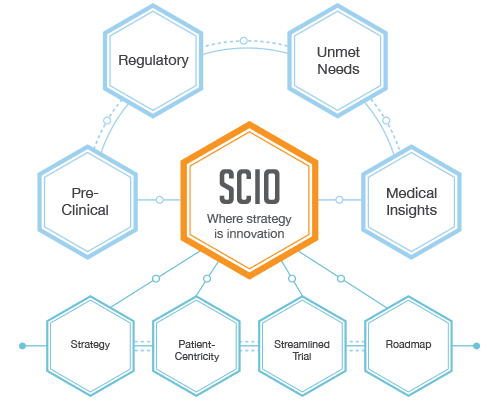
The Strategic Clinical Innovation Organization (SCIO) concept developed by BBCR was designed specifically to help pharmaceutical innovators address the concerns and maneuver around evolving challenges. SCIO allows for time and cost efficiencies, and risk mitigation.
The BBCR team is armed with extensive clinical, regulatory and industry experience that we use in creating a product specific clinical/regulatory strategy as part of the IND or IDE and before tactical clinical activities start. We aim to transform the transition stage between pre-clinical and clinical endpoints with smart strategy that, at a minimum, redirects the costs to benefit patients.
The FDA has been calling for a smarter, more innovative approach, and we believe SCIO is the integrative, multidisciplinary approach to deliver it.
The Approach
BBCR has developed an approach to meet the challenges of researchers and innovators seeking a clearer path to market.
Flexible Road-Map
Successful clinical development, for drug and medical device companies (small and large), requires an early strategy with a flexible road-map that makes it actionable and sustainable.
Robust Clinical Data
Customized Strategy and Road-Map allow for robust clinical data generation and informed decision-making.
Clear Goals
Setting clear goals is essential to managing risk and pre-empting challenges. The BBCR team identifies innovative solutions and strategies using scientific, medical, regulatory and clinical research experience.
Cost-Effective Trials
A customized clinical/regulatory strategy, roadmap and continuity plan pave the way for cost-effective trials.
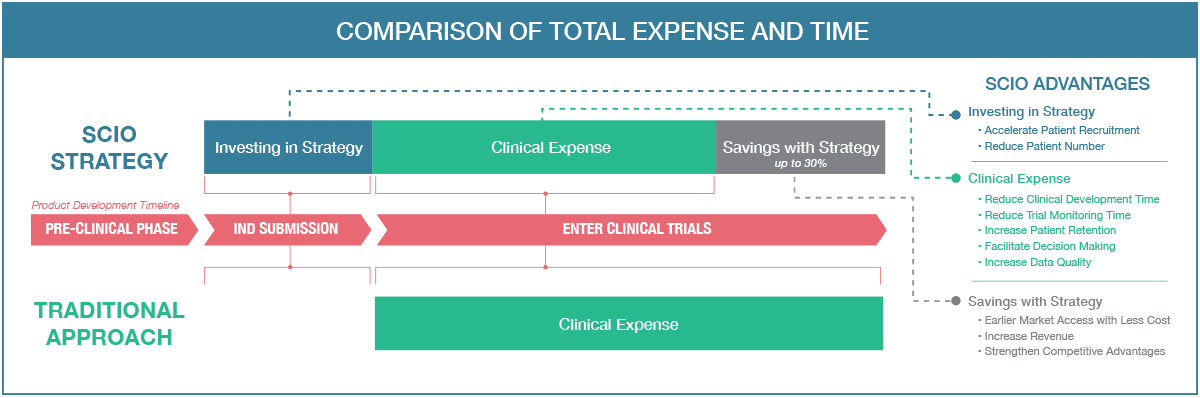
SCIO Advantages
Investing in Strategy
- Accelerate Patient Recruitment
- Reduce Patient Number
Clinical Expense
- Reduce Clinical Development Time
- Reduce Trial Monitoring Time
- Increase Patient Retention
- Facilitate Decision Making
- Increase Data Quality
Savings with Strategy
- Earlier Market Access with Less Cost
- Increase Revenue
- Strengthen Competitive Advantages

WE CREATE A ROADMAP CUSTOMIZED FOR YOUR PRODUCT
- Design-centric trials
- Patient-centric approach
- Simplified trials
- Streamlined development plan
- Cost efficiency
- Time efficiency
- Risk management

Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.