BBCR Voice
From the Perspective of Researchers, Clinicians, and Regulatory Experts
The most efficient path in the clinical research process is a moving target. Technology innovation and regulatory requirements require constant updates. Through BBCR Voice, we aim to share not only our knowledge and expertise but also solutions to current challenges. BBCR embraces the challenges of developers and investors seeking a more straightforward path to market.
Recent Posts
BBCR partners with clients to identify, develop, and advance a product’s unique strengths from early concept through to market. Our consultancy is grounded in deep expertise across cell, biologic, and gene therapies, with a particular focus on rare disease.
BBCR partners with sponsors and investors to navigate complex paths to market, strengthening product potential while advancing efficiency and safety. Through strategic expertise and early clinical research services, we support informed, timely decisions at every stage...
BBCR’s team of experts help clients match treatments to rare genetic conditions and unsolved diseases, then work collaboratively with a product developer on the best plan to market. To learn more, visit bbcrconsulting.com.
Did you know that about a third of orphan approvals by the FDA since the program began have been mostly for repurposed mass-market drugs? Drug repurposing acts to lower the need for early stage clinical trials and can help identify new uses for existing drugs. We...
Drug repurposing accelerates the path from discovery to the clinic, offering particular promise for rare, neglected, oncologic, and neurodegenerative diseases. Learn how we can help at bbcrconsulting.com
The BBCR consultants, experienced in orphan, rare and ultra-rare diseases, have advised biotech and venture capitalists for new indications evaluation and prioritization. BBCR’s team of industry experts can help match treatments to rare genetic conditions and unsolved...
Is Your Drug Development Strategy Built for Tomorrow’s Success?
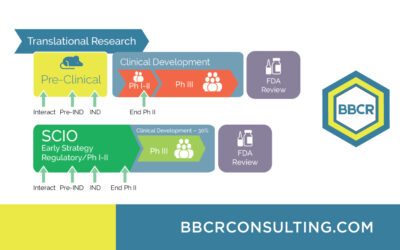
With BBCR’s Strategic Clinical Innovation Organization (SCIO℠) method, we’re redefining how innovation meets efficiency in drug development—providing a clear, data-driven path to market approval. The SCIO℠ method works by learning and predicting—analyzing each...
BBCR assists companies with the identification and adoption of biomarkers, now a routine part of drug development, and especially valuable in rare disease and precision medicine product development. Reach out to us to learn how we can assist with your development project.
The FDA recognizes biomarker development as a high priority area for future research. BBCR can help your company with your product development plan and validation. BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan...
Partnering with BBCR Consulting means gaining the support of a specialized team committed to making the clinical trial process more efficient.
Partnering with BBCR Consulting means gaining the support of a specialized team committed to making the clinical trial process more efficient. We deliver expert guidance in Orphan Drug Development by designing tailored clinical and regulatory roadmaps with simplified...
Who is BBCR and how do we help pharmaceutical innovators in the specialized rare diseases and orphan drug industries? We invite you to learn more at bbcrconsulting.com.
Our operational mission is to craft customized strategies that achieve cost-effective trials by 1) simplifying clinical plans, 2) streamlining trial protocols and 3) creating robust regulatory roadmaps for speed to market. BBCR's extensive experience in biologics for...
Accelerate Drug Development with Biomarker Strategy in Orphan Diseases and Precision Medicine
In the fields of orphan diseases and precision medicine, establishing a robust biomarker strategy during translational and early clinical development is key to expediting time-to-market. A well-defined biomarker plan not only streamlines regulatory approval but also...
At BBCR, our team of seasoned experts is dedicated to helping clients navigate the intricate landscape of rare genetic conditions and unsolved diseases. We work hand in hand with product developers to create the most effective strategies for bringing innovative treatments to market.
At BBCR, our team of seasoned experts is dedicated to helping clients navigate the intricate landscape of rare genetic conditions and unsolved diseases. We work hand in hand with product developers to create the most effective strategies for bringing innovative...
Our experienced CRO Management and Drug development team identify study remediation strategies and provide a resource for any Study Rescue. We invite you to reach out to learn more – visit bbcrconsulting.com.
Every clinical study has its unique challenges that initially may not have been factored for. Experienced management can help sponsors to address prolonged trial timeline and high quality data. Rare Diseases and Precision Medicine Require Unique Approaches In Clinical...
A pioneering figure in the area of rare diseases and orphan drug early-stage clinical research and regulatory strategies, Dr. Candida Fratazzi has an impressive 25-year track record in this specialized field.
Dr. Fratazzi's contributions have been significant. Her journey began when she founded BBCR Consulting, an organization dedicated to strategic clinical innovation, aiming to streamline the often convoluted processes of clinical trials. Drawing from her extensive 25...
BBCR is committed to cultivating and enhancing a product’s unique strengths at every stage, from initial conception to successful market launch. With deep expertise in cell therapy, biologics, and gene therapy—particularly in the realm of rare diseases—we provide strategic guidance and innovative solutions that form the foundation of our consultancy practice.
We are dedicated to addressing the complex challenges faced by sponsors and investors who seek a clear and streamlined path to market. Our approach focuses on nurturing the inherent strengths of their products while simultaneously enhancing efficiency, safety, and...
BBCR embraces innovative strategic consulting for highly effective clinical development planning and regulatory strategy. Reach out today to learn more about how we can help advance your project.
Our industry needs innovative strategies, and reduced-risk clinical trials. BBCR clinical development services and drug development consulting integrates real world evidence into clinical development plans and regulatory strategies. We ensure that focus goes towards...
Partnering with BBCR Consulting streamlines the clinical trial process by delivering tailored clinical and regulatory roadmaps. Our approach focuses on simplified programs and optimized protocols, designed to align seamlessly with each client’s unique requirements.
We invite you to read some of our Case Studies below Reduced Comparator High Cost for Biosimilars PROJECT An executive at a pharmaceutical company asked BBCR to review its biosimilar pre-clinical data and prepare the IND package. In addition to evaluate the...
BBCR meets clients’ needs in the fast paced and ever-changing regulatory environment. As specialists in Orphan and Personalized Medicine, BBCR helps clients identify areas of need or economic interest and helps them to find homes for treatments for rare diseases and precision medicine.
BBCR helps orphan drug developers find direction in clinical trials involving biologics, biosimilars, small molecules, medical devices, and repurposing. BBCR consultants have the experience to guide you through the development process with a clinical plan and a...
BBCR is highly experienced in developing innovative approaches to de-risk your product development during the early clinical development stage, including designing Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies. Learn more at bbcrconsulting.com #PoC #PoM #proofofconcept #proofofmechanism #earlyclinicaldevelopment #strategy
BBCR specializes in the strategy and delivery of early-phase clinical development services to enable informed, timely decision making for our clients. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose...
BBCR partners with small and medium sized drug and device biotechnology firms. Our clients are clinical researchers and innovators looking for an efficient path to approval, and come to us from across the globe. Learn more about our consulting services at bbcrconsulting.com.
The BBCR mission is to simplify clinical research, encourage cost-effective trials, and help innovators navigate through the regulatory process. Who We Serve: Small & Medium Sized Biotech Companies currently moving from pre-clinical studies toward clinical trials...
From Preclinical through Phase I and POC studies, BBCR provides early clinical research services to enable informed, timely decision-making for our clients. We offer clinical, regulatory, translational, and biomarker consulting services that support our clients’ needs. Our process is designed to maximize time and cost efficiencies while mitigating risk.
BBCR Consulting offers clinical, regulatory, translational, and biomarker consulting services that support our clients’ needs. Our process is designed to maximize time and cost efficiencies while mitigating risk. Our consultants have the experience to guide you...
With Biomarkers a routine part of drug development, BBCR assists companies with the identification and adoption of biomarkers, especially valuable in rare disease and precision medicine product development.
The FDA recognizes biomarker development as a high priority area for future research. BBCR can help your company with your product development plan and validation. BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan...
“The beginning is the most important part of the work.” — Plato. BBCR is dedicated to developing and nurturing a product’s unique strengths from conception to market. Our expertise with cell, biologics and gene therapy as they relate to rare disease is the cornerstone of our consultancy practice.
BBCR is committed to meeting the challenges of sponsors and investors seeking a clear path to market by nurturing their products’ strengths while improving efficiency and safety. We specialize in strategy and provide early clinical research services to enable...
Dr. Candida Fratazzi is a pioneering figure in the area of rare diseases and orphan drug early-stage clinical research and regulatory strategies. With an impressive 20-year track record in this specialized field, Dr. Fratazzi’s contributions have been significant.
Her journey began when she founded BBCR Consulting, an organization dedicated to strategic clinical innovation, aiming to streamline the often convoluted processes of clinical trials. Drawing from her extensive 25 years in biomedical research, Dr. Fratazzi introduced...
BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan drug indications by developing and nurturing the product’s unique strengths. Our operational mission is to craft customized strategies that achieve cost-effective trials. Reach out today to learn more.
BBCR’s mission is to deliver cost-effective clinical plans and regulatory strategies for cell and gene therapy programs in the areas of rare disease. Cell and Gene therapy are the new frontiers in the fight against devastating diseases, including rare diseases and...
BBCR provides Orphan Drug solutions that empower sponsors with medical insight, ODA application experience, strategic clinical planning, and streamlined trial design.
Our selected services include expertise with Biomarkers & Surrogate Endpoints, Regulatory Affairs FDA & EMA, Medical Affairs & Clinical Research, Due Diligence, and Trial Management & Trial Rescue. BBCR's Strategic Clinical Innovation OrganizationSM...
By collaborating with Boston Biotech Clinical Research, you’ll be working with a team of experts who are dedicated to streamlining the clinical trial process.
We provide expert guidance for Orphan Drug Development by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. BBCR is dedicated to supporting pharmaceutical innovators in the specialized...
BBCR’s mission is to deliver cost-effective clinical plans and regulatory strategies for cell and gene therapy programs in the areas of rare disease. We invite you to learn more at bbcrconsulting.com.
Cell and Gene therapy are the new frontiers in the fight against devastating diseases, including rare diseases and cancers. Clinical trial results have been promising, and the next generation of Cell and Gene therapies holds tremendous promise for many patients and...