Emerging cell and gene therapy may offer sustained long-term correction for LSD patients
Dr. Maria Niu
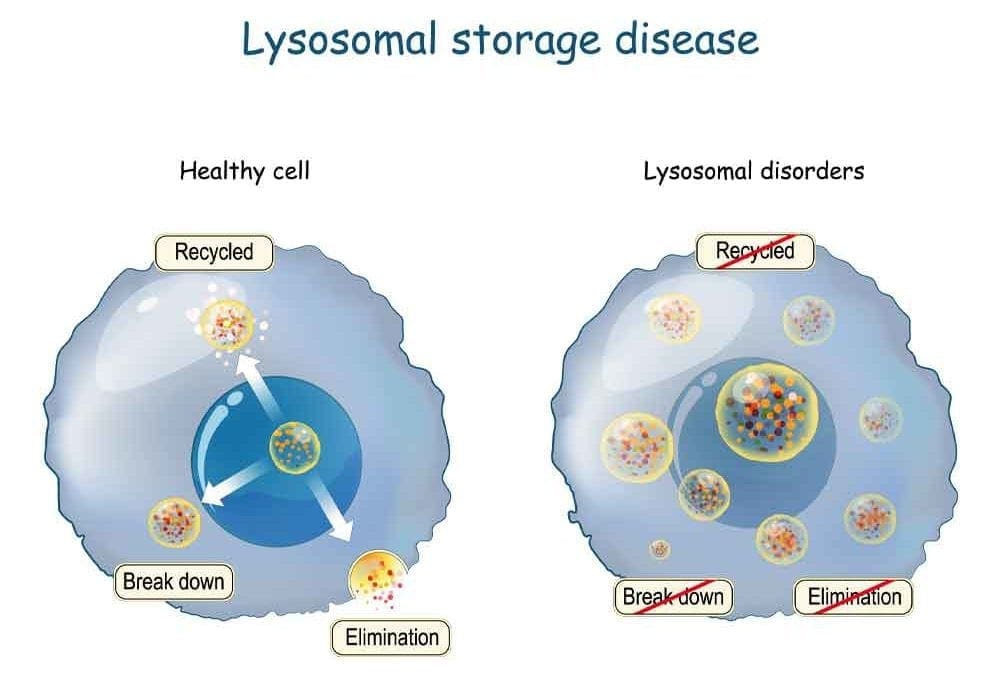
Lysosomal storage diseases (LSDs) are rare inherited metabolic diseases and characterized by the accumulation of substrates in excess in various organs’ cells due to lysosomes’ defective functioning.
The combined incidence of LSDs is between 1 in 5000 to 1 in 10000. LSDs occur worldwide, but some types of diseases have a higher incidence in a particular population.
The treatment of LSDs mainly includes Enzyme Replacement Therapy (ERT), Pharmacological Chaperone Therapy (PCT), and Small Molecule Assisted Substrate Transportation (SMAST).
In recent years, Hematopoietic Stem Cell Transplantation (HSCT) and Gene Therapy (GT) have come to our attention and developed gradually.
According to the data from www.clinicaltrials.gov, more than seven hundred interventional studies target LSDs; among them, ~14% of studies focus on HSCT and GT. Although the challenges exist, cell and gene therapy are rising, with the FDA accelerating approvals and some treatments already on the market.
Moreover, based on the latest clinical research report https://pubmed.ncbi.nlm.nih.gov/31056978/, the adeno-associated virus vector-mediated gene or gene-corrected/modified stem cells could reverse the metabolic defects associated with LSD and reduce inflammation in LSD, leading to a clinical benefit.
We have worked on efficacy and safety evaluations and seen positive results in numerous pre-clinical studies and promising clinical studies.
We are expecting an excellent prospect on cell and gene therapy in the LSDs field.
#genetherapy #celltherapy #LSD #Lysosomalstoragediseases #clinicalstudies #efficacyevaluations #safetyevaluations

Specializing in rare disease, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.