
Tagged: Cost effective trials
The SCIO concept is the value proposition solution for cost-effectiveness and risk management in clinical development. Learn more about how BBCR can help.
March 9th, 2022 | Cost effective trialsBoston Biotech Clinical Research (BBCR) works with biotech, pharmaceutical, and device companies to develop a clinical and regulatory roadmap consisting of simplified clinical programs, streamlined protocols, and cost-effective trials.
February 22nd, 2022 | Cost effective trials
Our Boston-based, integrated, boutique consulting team specializes in rare disease and orphan indications, and is dedicated to supporting pharmaceutical innovators and nurturing each product’s strengths. The BBCR mission is to simplify clinical research, encourage cost-effective trials, and help innovators navigate through the regulatory process.
The Strategic Clinical Innovation Organization (SCIO) concept developed by BBCR was designed specifically to help pharmaceutical innovators address the concerns and maneuver around evolving challenges. SCIO ensures time and cost efficiencies, and risk mitigation.
June 10th, 2021 | Cost effective trials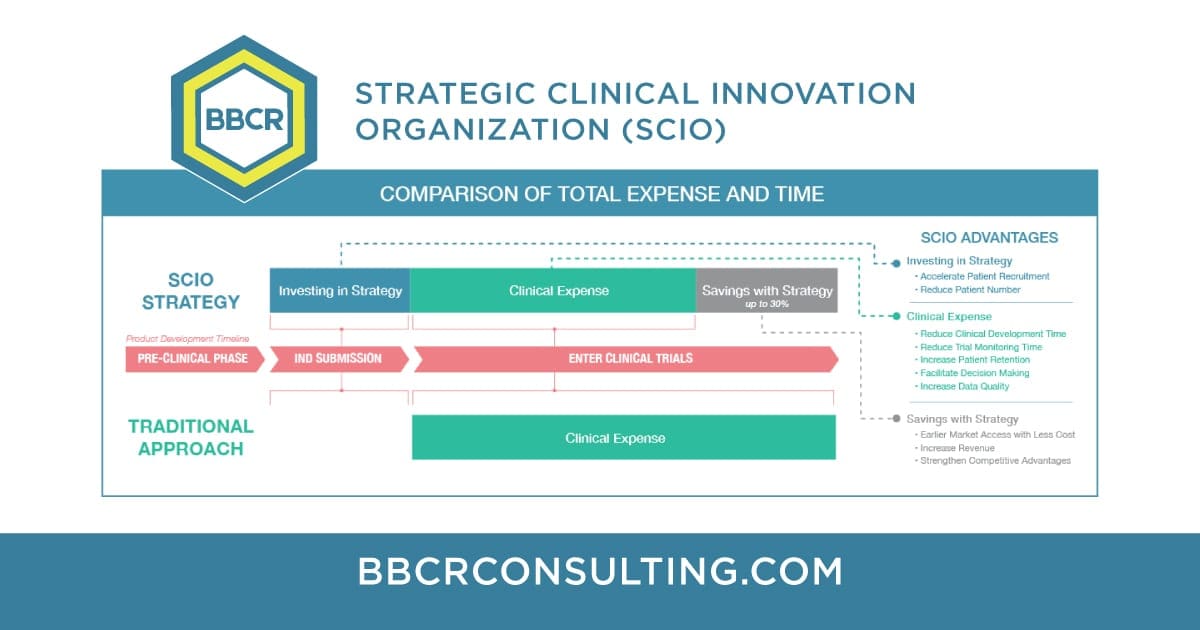
BBCR has developed an approach to meet the challenges of researchers and innovators seeking a clearer path to market. Flexible Road-Map Successful clinical development, for drug and medical device companies (small and large), requires an early strategy with a flexible road-map that makes it actionable and sustainable. Robust Clinical Data Customized Strategy and Road-Map allow […]
