BBCR Voice
From the Perspective of Researchers, Clinicians, and Regulatory Experts
The most efficient path in the clinical research process is a moving target. Technology innovation and regulatory requirements require constant updates. Through BBCR Voice, we aim to share not only our knowledge and expertise but also solutions to current challenges. BBCR embraces the challenges of developers and investors seeking a more straightforward path to market.
Recent Posts
Why Bacterial Consortia May Stop C. diff. Recurrences – Dr. Candida Fratazzi, MD interviewed on Voice America for the C Diff Foundation
We were pleased to be invited by Nancy C. Caralla of the C Diff Foundation to this important discussion on the Voice America Radio Network about how Microbiome functions and how its changes impact our health. [button...
Pediatric Rare diseases and Rare Pediatric Disease Designation – Boston Biotech Clinical Research
Pediatric Rare diseases and Rare Pediatric Disease Designation FDA will award priority review vouchers to sponsors of certain rare pediatric disease product applications that meet the criteria specified. Section 529 of the FD&C Act is intended to encourage...
BBCR Consulting will attend the World Orphan Drug Congress USA 2020 August 24 – August 26
The World Orphan Drug Congress USA will be a live virtual conference. BBCR’s Team invites you to send an email at info@nisse.serpcom.com/bbcr2. We hope to meet many of you that will attend the World Orphan Drug Congress. We are very much interested in learning about...
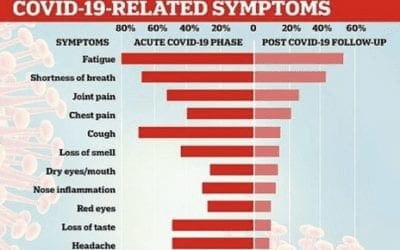
Long-Term effects of COVID 19 infection
Patients with mild COVID 19 infection, from a variety of age groups, were still experiencing major symptoms for months after COVID 19 disease is over. Read full article: https://jamanetwork.com/journals/jama/fullarticle/2768351 The Jama study was conducted in 143...
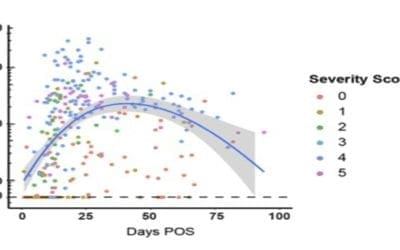
Decline of antibody (Ab) anti Covid19 within 60 days post infection
A transient neutralizing Ab (nAb) response is a feature shared by both COVID19 infection and the coronaviruses that cause common colds (https://www.medrxiv.org/content/10.1101/2020.07.09.20148429v1) Antibody (Ab) responses to SARS-CoV-2 can be detected in most...
Airborne SARS-CoV-2 Transmission – World Health Organization addresses the potential for airborne transmission of the coronavirus
SARS-CoV-2 outbreaks that cannot be explained by large droplets and contact with surfaces alone. Droplets from speech can float in air for eight minutes. A growing body of evidence suggests beyond any reasonable doubt that the virus spreads indoors through tiny...
Severe COVID-19 linked to a Genetic Region
Scientists launched studies in search of genes that could explain why some people infected with SARS-CoV-2 get really sick, while others have only mild symptoms. We know that chronic health conditions—such as hypertension and diabetes can play a role, but there are...
Developing Drugs Able to Treat Neurological Disease – Boston Biotech Clinical Research
Techniques that bypass or trick the guardian of the central nervous system. Developing drugs that can penetrate into the brain requires some creativity. The approaches vary, each technique comes with its benefits and drawbacks, making it appropriate for some patients...
Early loss of COVID-19 antibodies! …Plus hope for good news from the several vaccines currently in development.
Preliminary data suggest that survivors of SARS-CoV-2 infection may be susceptible to reinfection within weeks or months. One study in ~1,500 COVID patients show that 10 percent % of these patients had undetectable antibody levels within weeks of first symptoms or...
Artificial intelligence Diagnostic tool for COVID-19 and Neurodegenerative Diseases
Lesions in the lungs of patients with pneumonia caused by a SARS-CoV-2 infection are distinct from those caused by bacteria. SARS-CoV-2 is known to damage lung tissue as many COVID-19 patients develop pneumonia, which can progress to respiratory failure and sometimes...
Drug repurposing acts to lower the need for early stage clinical trials and can help identify new uses for existing drugs.
People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. One attractive option of Drug Repurposing is to use a scientific approach to identify new uses for existing drugs. About a third of...
Biologic treatments show promise in providing clinical solutions to a variety of diseases. BBCR can assist with the development of a targeted strategy to meet your study needs.
BBCR's team has experience in biologics for rare diseases including rare cancers and precision medicine and can help develop a targeted strategy including studies with fewer patients to control safety issues. Services include: Indications analysis and prioritization...
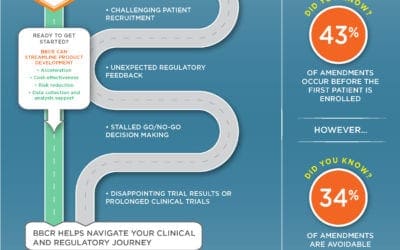
BBCR Streamlines Clinical Development with the Clinical and Regulatory Expertise to Support Your Development Needs
BBCR Consulting offers world-class regulatory, clinical research, and biomarker consulting services that provide high-value, and support our clients’ operational and functional needs. Our process is designed to maximize time efficiencies, risk mitigation, and cost...
The future of Orphan Drug Clinical Trials
Biotech companies' continued participation in the Global Orphan Drug Market Opportunity requires the development of new strategies rooted in the established knowledge of orphan developer experts. Participation of big pharmaceutical companies in the ongoing clinical...
First Coronavirus deaths reported in indigenous communities that live in Amazonia (National Geographic).
At least 28 communities living in extreme isolation in the Brazilian Amazon, and there may be as many as 80 more, have confirmed to be in existence. On April 9, a Yanomami adolescent had died of COVID-19 in the northern state of Roraima. The youth had moved back and...
Jump start Clinical Research after COVID 19
Given the global Covid19 emergency and the global nature of the pharmaceutical and biotech industry, key operational areas will be impacted. As with any emergency response, the key to management is implementing mitigation plans. Early stage pharmaceutical and biotech...
FDA approved mesenchymal stem cell (MSC) treatments as compassionate use in the very sickest COVID-19 patients.
A pilot study in China in which seven COVID-19 patients received intravenous infusions of donor mesenchymal stem cells (MSC) indicates that the intervention was safe, and that the approach may improve infection outcomes was reported in Aging and Disease last month. We...
COVID-19 four vaccine candidates have been approved for early testing in people.
Four vaccine candidates—two in the US, one in China, and one in the UK—have been approved for early testing in people. Many others are also following. Researchers look to messenger RNA encased in nanoparticles, DNA plasmids, molecular clamps, and other approaches as...
Saliva Test for COVID-19 reduces health care professional risk of infection
Saliva Test for COVID-19 Approved for Emergency Use by FDA on 13 April 2020. Saliva testing will help with the global shortage of swabs, increase possibility of people testing, and reduce risk to collect samples by health care professionals. The saliva-based test...
COVID-19 Global Pandemic disruption in RARE DISEASE clinical research
The COVID19 has taken the world by a stranglehold and the outbreak is causing significant disruptions in clinical research which is facing significant challenges. The process for conducting conventional clinical trials become difficult and risky at a time when the...
Protective Antibodies to COVID-19
Whether people develop immunity to COVID19 after being infected once is a pressing question. It’s of particular interest to several research developing plasma therapies, whereby antibody-containing blood plasma is extracted from recovered patients and administered to...
COVID19 Infects the Nervous System
The fact that COVID-19 patients have lost their sense of smell or taste is interesting because, if the virus infects the nose, it would use the exact same neurons as in the mouse studies to enter the brain. SARS‐CoV2 causes epidemic pneumonia characterized by acute...
Clinical Studies: COVID-19 Gut Infection in Pediatrics
Several studies were conducted to evaluate the presence of COVID-19 in the GI track and eliminated with stools. The first case report in the USA of COVID-19 was published in The New England journal of medicine (https://www.nejm.org/doi/full/10.1056/NEJMoa2001191). The...
Clinical Trial of COVID-19 Vaccine Begins
The first participant in a clinical safety trial for a COVID-19 vaccine conducted by the Kaiser Permanente Washington Health Research Institute in Seattle was given an experimental dose on March 16, reports the Associated Press. Testing will begin with 45 young,...
How soon can a vaccine can be tested in Clinical trials? #clinicaltrials
How do viral genomes’ sequences from swabs taken from infected patients help you build a family tree of the virus? Random mutations in the SARS-CoV-2 pathogen’s genome help researchers track the spread and transmission of COVID-19, the disease it causes. Source: News...