BBCR Voice
From the Perspective of Researchers, Clinicians, and Regulatory Experts
The most efficient path in the clinical research process is a moving target. Technology innovation and regulatory requirements require constant updates. Through BBCR Voice, we aim to share not only our knowledge and expertise but also solutions to current challenges. BBCR embraces the challenges of developers and investors seeking a more straightforward path to market.
Recent Posts
Clinical trials are time-consuming, expensive, and often burdensome on patients. Clinical trials can fail for many reasons. Our experience provides an understanding of these reasons and offers insights into opportunities for improving the likelihood of creating and executing successful clinical trials.
The Trial Management That Addresses Risks Experienced management address risks such as: Delayed enrollment Multiple protocol amendments Poor patient retention Poor data quality Prolonged trial completion timeline Poor safety monitor Let BBCR Consulting assist in your...
BBCR is attending this week’s SITC 37th Annual Meeting & Pre-Conference Program and we will reporting on conference events soon.
The Society for Immunotherapy of Cancer’s (SITC) 37th Annual Meeting & Pre-Conference Programs (SITC 2022) are taking place Nov. 8–12, 2022, at the Boston Convention and Exhibition Center in Boston. BBCR will be attending the conference this week and is looking...
We urge caution when it comes to planning to submit your PRE-IND or IND meeting request so as to avoid common mistakes. We can help with your development plan– contact BBCR to learn more.
Rare disease drug development requires knowledge and experience that is not always intuitive or deductible from large population drug development experience. Looking for a Rare Disease drug development expert, both in regulatory and medical affairs, is highly...
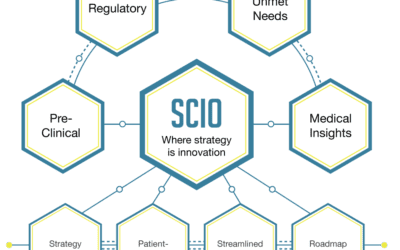
SCIO assists with early stage biotech company evaluation. Learn why adopting this clinical roadmap and development strategy is critical to your success in bringing your product to market.
How can SCIO SM help? To enhance your position, it becomes crucial to demonstrate that your product/technology is the “safe” option for investment and a stand-out opportunity by including a clinical roadmap and development strategy of your product/technology. This...
Efficiencies in clinical research have become essential in the areas of trial management and trial rescue
Every clinical study has its unique challenges that initially may not have been factored for. Experienced management can help sponsors to address prolonged trial timeline and data quality. Rare Diseases and Precision Medicine require unique approaches to trial...
BBCR specializes in taking the complexity inherent to the regulatory phase of the approvals and simplifying it by strategizing our support to each client based on product-specific regulatory requirements and functional needs.
BBCR can assist clients in the following areas: Regulatory Strategy Thinking ahead during the regulatory process, we avoid unnecessary hurdles in the approvals process. We identify gaps, work through the right strategies for an effective product application, and...
Orphan drug designation provides key incentives of the ODA, including significant tax credits for qualified clinical testing. The BBCR team will empower sponsors with medical insight, ODA application experience, strategic clinical plan, and streamlined trials design.
Developing drugs with substantial benefits in smaller, molecularly defined, pharmacologically relevant subpopulations of patients with the same clinically recognized disease is increasingly being viewed as a viable pathway for bringing drugs to market. [button...
BBCR supports innovative and repurposing treatments for orphan diseases.
Developing innovative or repurposing drugs for orphan diseases can be rewarding, but navigating the challenges is not for the faint of heart. Expert guidance is essential in an area where patients are few; a lack of previous studies may hamper progress as you mount an...
BBCR provides expert development guidance for Orphan Disease. The BBCR mission is to customize strategies, simplify clinical research, design cost-effective trials, streamline protocols, and create a regulatory roadmap.
As one of the first consultancy teams to streamline clinical trials, BBCR’s boutique consulting team specializes in rare disease and orphan indications dedicated to supporting pharmaceutical innovators, and nurturing each product’s strengths. The BBCR Team provides...
BBCR offers Translational Medicine and Biomarkers Program Consultation to bridge the gap between pre-clinical and early clinical development
Translational medicine is a bridge between pre-clinical and early clinical development (from bench to bedside), creating the foundation for precision medicine in late clinical development. A translational medicine program includes the adoption of biomarkers to...
The BBCR mission is to customize strategies, simplify clinical research, design cost-effective trials, streamline protocols, and create a regulatory roadmap. Learn more about our services by visiting bbcrconsulting.com
How we can help The BBCR team provides knowledge in cell, biologics and gene therapies BBCR addresses sponsors' questions in the ever-changing regulatory environment We offer clinical strategy for orphan diseases and precision medicine RWE can be used to build orphan...
BBCR regularly partners with small and medium sized drug and device biotechnology firms. Our clients are innovators and clinical researchers looking for a more efficient path to approval, and come to us from across the globe. Reach out today to learn more.
The BBCR mission is to simplify clinical research, encourage cost-effective trials, and help innovators navigate through the regulatory process. Small & Medium Sized Biotech Companies currently moving from pre-clinical studies toward clinical trials in need...
BBCR’s team of experts is highly experienced in the areas of Real World Evidence (RWE) for Control Arm, Post-approval Requirements and Disease Natural History. Reach out today to learn more.
Real-world evidence (RWE) is increasingly important in clinical trial design and drug approval. RWE is clinical evidence related to patient health status and treatment safety and efficacy that are generated in clinical practice. The FDA developed a framework to use...
BBCR specializes in strategy and early clinical research services from preclinical through Phase I and POC studies to enable informed, timely decision making for our clients.
BBCR Consulting offers clinical, regulatory, translational, and biomarker consulting services that support our clients' needs. Our process is designed to maximize time and cost efficiencies while mitigating risk. We partner with domestic and international companies....
BBCR’s experienced CRO Management and Drug development team identify study remediation strategies and provide a resource for any Study Rescue
Every clinical study has its unique challenges that initially may not have been factored for, presenting the need for study remediation and rescue. Experienced management can help sponsors to address prolonged trial timeline and high quality data. Rare Diseases and...
An attractive option of drug repurposing is to use a scientific approach to identify new uses for existing drugs thereby reducing the need for early stage clinical trials.
People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. About a third of orphan approvals by the FDA since the program began have been mostly for repurposed mass-market drugs. BBCR’s team of...
BBCR meets clients’ needs in the fast paced and ever-changing regulatory environment. As specialists in Orphan and Personalized Medicine, BBCR helps clients identify areas of need or economic interest and helps them find homes for treatments for rare diseases and precision medicine.
BBCR helps orphan drug developers find direction in clinical trials involving biologics, biosimilars, small molecules, medical devices, and repurposing. BBCR consultants have the experience to guide you through the development process with a clinical plan and a...
BBCR offers expertise in the areas of Product Development Planning and Validation or Qualification when biomarkers are a component of your product development plan.
How BBCR Can Help BBCR’s biomarker expert is renowned in the field. He is a senior advisor on the biomarker committee at the NIH and former global biomarker expert at Novartis, Roche, and Pfizer.Insightful strategy is the most effective way to ensure quality when...
We are pleased to announce that BBCR Consulting President, Candida Fratazzi, MD will be the keynote speaker at the upcoming 9th Clinical Trials Strategic Summit happening in San Francisco on October 27th and 28th, 2022
BBCR is looking forward to attending this important event and expresses gratitude to Agile Falcon Strategic Group LLC for the opportunity to be a part of the upcoming Summit. The 9th Clinical Trials Strategic Summit (CTSS) will be taking place in San Francisco October...
BBCR specializes in rare disease, working with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process in order to achieve optimal product market positioning.
Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. Click the button below to learn more about how we have helped...
Strategic Consulting and the SCIO Process – Paving the Way for Drug Development
SCIO is critical for accelerating approval and significantly reducing the costs of drug and medical device development. One of the major challenges facing this industry is the rising cost of clinical trials. Thus, it is crucial to ensure that trials are significantly...
Why choose BBCR for your product development needs?
BBCR can help streamline product development and help to successfully navigate your clinical and regulatory journey. BBCR focuses on: Acceleration Cost-effectiveness Risk reduction Data collection and analysis support [button link="https://bbcrconsulting.com/"...
BBCR can help medical device companies with clinical data strategy from conception to market, from preparing due-diligence ready datasets for funders to post-marketing safety surveillance for regulatory authorities.
Medical devices have their own special complications as it relates to clinical trials. We work closely with each client to bring device innovation to market faster and more affordably. Working with the BBCR team will help that streamlines clinical trials and help you...
BBCR has extensive experience in early development Phase 1 and 2, from pre-clinical to pre-IND. BBCR’s early drug development experience includes the development of clinical strategy and plan, including target product profiles and clinical protocols.
Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process and strategy. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road...
BBCR’s team of professionals can help with your Pre-ind Integrated Development Plan
A strategy that clearly marks the path to success creates opportunity to reduce risk. BBCR works with clients to achieve a successful pre-IND meeting by: Reviewing scientific data Setting regulatory strategy Setting integrated plan Creating messages and outlines...