BBCR Voice
From the Perspective of Researchers, Clinicians, and Regulatory Experts
The most efficient path in the clinical research process is a moving target. Technology innovation and regulatory requirements require constant updates. Through BBCR Voice, we aim to share not only our knowledge and expertise but also solutions to current challenges. BBCR embraces the challenges of developers and investors seeking a more straightforward path to market.
Recent Posts
BBCR’s mission is to deliver cost-effective clinical plans and regulatory strategies for cell and gene therapy programs in the areas of rare disease. We invite you to learn more at bbcrconsulting.com.
Cell and Gene therapy are the new frontiers in the fight against devastating diseases, including rare diseases and cancers. Clinical trial results have been promising, and the next generation of Cell and Gene therapies holds tremendous promise for many patients and...
BBCR uses innovative approaches to de-risk your product development. For our clients interested in Proof of Concept and Proof of Mechanism – PoC and PoM – Our team has the expertise to ensure successful product development at any stage of development.
The BBCR team designs Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies with the drug clinical plan and regulatory strategy in mind. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose...
Why a Strategy Plan Before First in Human
Innovation in clinical research is a long-term need due to several factors, including the high failure rate and skyrocketing costs. Healthcare has been changing rapidly for many years. The COVID-19 pandemic has accelerated by helping drive the shift to value-based...
Why and how to invest in Early Strategy before First in Human
Innovation in clinical research is a long-term need due to several factors, including the high failure rate and skyrocketing costs. Several factors contribute to the skyrocketing cost of clinical trials. Integration of advanced technologies into drug development....
BBCR is committed to meeting the challenges of sponsors and investors seeking a clear path to market.
Our mission is to support domestic and international pharma, biotech, and device companies by nurturing their products’ strengths while improving efficiency and safety. We specialize in strategy and provide early clinical research services to enable informed, timely...
There remains continued interest in the use of surrogate endpoints as primary measures of the effectiveness of investigational drugs in definitive drug trials. BBCR’s published White Paper – Surrogate Endpoints for an Accelerated Orphan Drug Approval – remains a valuable resource in the area of orphan drug development.
Rare Diseases are conditions that affect less than 200,000 people in the U.S. However, over 7,000 Rare Diseases affect more than 400,000,000 people worldwide, including ~25 million in the U.S. and ~30 million in Europe (1). In recent years, the number of orphan drugs...
Early Clinical Strategy Mistakes to Avoid
While an early clinical strategy offers numerous advantages, it also comes with certain challenges that need to be considered: Limited Information: Limited information available about the investigational product can make it challenging. High Risk of Failure:...
An Early Clinical Strategy Includes Several Key Elements
Developing an effective early clinical strategy requires collaboration between various experts. The strategy is a living document that can evolve as new data becomes available and as the project progresses through clinical development. Study Design: The design must be...
Recognize the Benefit of an Early Clinical Strategy
Early clinical strategy refers to the comprehensive plan and approach that pharmaceutical companies, biotech firms, and researchers develop for the initial phases of clinical development of a new drug. These early phases, typically known as Phase 1 and Phase 2...
Early Clinical Strategy: Portfolio Expansion Indication Selection
Expanding a product portfolio through the selection of additional indications can offer strategic growth opportunities. However, the process of selecting indications for portfolio expansion requires careful consideration of various factors. Here are few of them for...
Collaborating with Boston Biotech Clinical Research can streamline the clinical trial process. We customize a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. We invite you to learn more at bbcrconsulting.com.
Providing Expert Guidance for Orphan Drug Development BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan drug indications by developing and nurturing the product’s unique strengths. Our operational mission is to craft...
Orphan drug designation provides critical incentives for the ODA, including significant tax credits for qualified clinical testing. Learn more about Precision Medicines’ Impact on Orphan Drug Designation or contact BBCR today at (617) 401-2327.
The BBCR team will empower Sponsors with medical insight, ODA application experience, strategic clinical planning, and streamlined trial design.
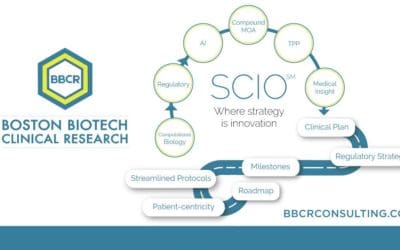
The Strategic Clinical Innovation Organization (SCIO) method is explicitly designed to help pharmaceutical innovators address their concerns and maneuver around evolving challenges. SCIO identifies time and cost efficiencies and relief from risk management on their journey to market approval. Reach out today to learn more.
An early-phase strategy improves productivity and the path to market approval. SCIO SM Advantages Accelerate Patient Recruitment Reduce Patient Number Reduce Clinical Development Time Reduce Trial Monitoring Time Increase Patient Retention Facilitate Decision Making...
BBCR is highly experienced in developing innovative approaches to de-risk your product development during the early clinical development stage, including designing Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies. Reach out to us at bbcrconsulting.com to learn more.
BBCR specializes in the strategy and delivery of early-phase clinical development services to enable informed, timely decision making for our clients. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose...
Interest is increasing rapidly in using surrogate endpoints as primary measures of the effectiveness of investigational drugs in definitive drug trials. Learn more at bbcrconsulting.com.
The primary difference between a biomarker and a surrogate endpoint is that a biomarker is a “candidate” surrogate marker. In contrast, a surrogate marker is a test used and taken to measure the effects of a specific treatment. In orphan diseases and precision...
BBCR has extensive experience in early development Phase 1 and 2 process and strategy, from pre-clinical to pre-IND. Our early drug development experience includes the development of clinical strategy and plan, including target product profiles and clinical protocols.
Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process and strategy. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road...
BBCR has extensive experience in biologics for rare diseases and can assist with the development of a targeted strategy to meet your study needs. Reach out today to learn more.
Cell and Gene therapy are the new frontiers in the fight against devastating diseases, including rare diseases and cancers. Strategies that move products to market faster: A cost-effective Clinical Plan that saves time and resources Medical Monitoring Ad-interim Chief...
BBCR has many useful resources on our website that highlight our case studies and offer timely and informational news and content via our blog.
In addition, our Resources section includes published studies and conference updates that highlight the breadth and depth of our experience in the areas of orphan diseases. BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and...
The BBCR mission is to deliver cost-effective clinical plans and regulatory strategies for cell and gene therapy programs. We invite you to learn more at bbcrconsulting.com.
BBCR’s boutique consulting team specializes in rare diseases and orphan indications and dedicated to supporting pharmaceutical innovators, and nurturing each product’s strengths. The BBCR team provides consultancy in cell, biologics, and gene therapies BBCR addresses...
With Biomarkers now a routine part of drug development, BBCR works with companies in assisting with the identification and adoption of biomarkers, especially valuable in rare disease and precision medicine product development.
The FDA recognizes biomarker development as a high priority area for future research. BBCR can help your company with your product development plan and validation. [button...
BBCR is highly skilled to meet clients’ needs in the fast paced and ever-changing regulatory environment. As specialists in Orphan and Personalized Medicine, BBCR helps clients identify areas of need or economic interest and helps them find homes for treatments for rare diseases and precision medicine.
BBCR helps orphan drug developers find direction in clinical trials involving biologics, biosimilars, small molecules, medical devices, and repurposing. BBCR consultants have the experience to guide you through the development process with a clinical plan and a...
In orphan diseases and precision medicine, developing a biomarker plan during translation into early clinical development moves treatment to market faster, and still meets the FDA’s expectations.
Biomarkers and Surrogate Endpoints – Learn more about how BBCR can help get your product to market more efficiently with proper plan development and strategy. Interest is increasing rapidly in using surrogate endpoints as primary measures of the effectiveness of...
BBCR is honored to have contributed to the work, Bacterial Consortium Therapy for Prevention of Recurrent C difficile Infection, published April 15, 2023 in the JAMA Medical Journal.
The microbiome is the collection of genomes, genes, and gene products of the microbiota living in a given environment, such as a soil patch or the gut of a human being. While bacteria are the most prominent players, we also host single-celled organisms known as...
BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan drug indications by developing and nurturing the product’s unique strengths. We invite you to learn more about our services at bbcrconsulting.com.
Specializing in rare diseases, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process. Our experienced team helps clients reach their goals by customizing a clinical and regulatory...
Trial failures can arise from factors such as failing to maintain suitable protocols, being unable to follow FDA guidance, complex procedures for participating patients, slow recruitment, lack of efficacy, safety issues, and/or insufficient funding to complete a trial. BBCR experts provide an understanding of the reasons a trial failed and offer insights to create and execute successful clinical trial management protocols and CRO management.
Trial Management that addresses risks The experienced trial management address risks such as: Scarce enrollment Multiple protocol amendments Poor patient retention Poor data quality Prolonged trial completion timeline Poor safety monitor [button...