BBCR Voice
From the Perspective of Researchers, Clinicians, and Regulatory Experts
The most efficient path in the clinical research process is a moving target. Technology innovation and regulatory requirements require constant updates. Through BBCR Voice, we aim to share not only our knowledge and expertise but also solutions to current challenges. BBCR embraces the challenges of developers and investors seeking a more straightforward path to market.
Recent Posts
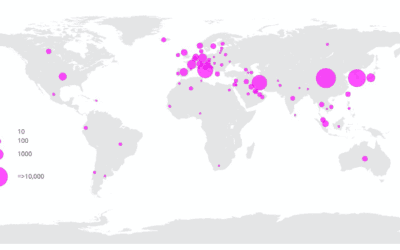
The worldwide spread of COVID-19 – How soon we will have Clinical trials?
Confirmed cases reported from each country so far. Data from cruise ship outbreaks not included. SOURCE: ECDC
How to shape clinical plan and study design to test how microbiome shapes immunity.
The gut microbiome serves many useful functions in the body, but it can also rev up the immune system in harmful ways. Zit has been pustulated that Diet can influence the microbiome and the mucosal immune response. In the paper: “Diet modulates colonic T cell...
Innovative therapies clinical plan requires understanding Why Immune Cells Extrude Webs of DNA and Protein
Today, it is widely accepted that NETs have both a protective and a pathological impact on the host. When neutrophils encounter pathogens, not only engage in phagocytosis and degranulation, they also release neutrophil extracellular traps (NETs) In CANCER,...
Clinical plan to test reconstitution of a normal gut microbiome in atopic dermatitis
Over the past 50 years, the frequency of allergies and autoimmune diseases has risen rapidly. Unfortunately, there hasn’t been much progress in understanding this epidemic of allergic disease. Hay fever and atopic dermatitis have both increased more than two-fold in...
Innovative Approach to Clinical Plan: Virtual Trial Design.
Health app designed to engage users through increased healthful living styles are at the base of this innovation in clinical plan and trial design. The design of the Health app was a collaborative effort between Janssen Pharmaceuticals and Apple Watch. The randomized...
Clinical plan and trial design challenges to develop new cancer treatment that modulate neutrophil extracellular traps (NETs)
Neutrophils use an enzyme called neutrophil elastase (NE) to cleave bacteria. Human neutrophils release NE which looks like a fibrous structure like webs. These webs, able to trap bacteria, are called neutrophil extracellular traps (NETs). These webs are constituted...
Head off the 2019-nCoV pandemic: Clinical plan for a possible therapy.
We’ve had a few events where they’ve jumped from animals into people. There’s a lot being done on how coronaviruses infect people from animals. We know the real key is to know what the host cell receptor is—that’s the protein on the surface of cells that viruses bind...
2019 Successful Clinical Plan and Drug Development Stories
Gene therapies for disorders such as X-linked Severe Combined Immunodeficiency (SCID, sometimes known as "bubble boy disease"), and Spinal Muscular Atrophy (SMA) have, for the first time, shown remarkable safety and efficacy results in clinical trials. FDA approved...
Experience in rare disease and enzyme replacement treatment may help clinical plans for Parkinson’s disease: Lysosomal Dysfunction – A Parkinson’s-Gaucher Link?
Worldwide, over 4,000 patients with Gaucher disease have received enzyme replacement treatment (ERT), which is safe and well tolerated. Gaucher disease is a rare disease caused by mutations in GBA1. GBA1 mutations drive extensive accumulation of glucosylceramide (GC)...
Potential Clinical Plans for Gene Therapies in Rare Disorders
BBCR Consulting, for the first time this year, decided to attend the European edition of the Gene Therapies in Rare Disorders conference in London UK. In our opinion, the event, as promised, proved to be: ”...uniquely focused conference that will bring the leaders in...
Challenges in autoimmune disease clinical plans
Autoimmunity occurs when the body is unable to differentiate “self” from “non-self” which results in overactive immune response against own cells and tissues. Autoimmune diseases affect 5 %-8% of the population; 78% affected are females. Low level autoimmunity is...
BBCR’s Innovation strategy consulting includes a clinical development plan service integrated into regulatory strategy which is supported by the proprietary SCIO method
A Clear Path to Approval The Strategic Clinical Innovation Organization (SCIO) concept developed by BBCR was designed specifically to help pharmaceutical innovators address the concerns and maneuver around evolving challenges. SCIO allows for time and cost...
The BBCR team designs Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies with the drug clinical plan and regulatory strategy in mind.
Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose for PoC, disease model and biomarkers Investigate drug concentration at the target site of action Investigate drug engagement with target molecular...
Our experienced CRO Management and Drug development team identify study remediation strategies and provide a resource for any Study Rescue
Every clinical study has its unique challenges that initially may not have been factored for. Experienced management can help sponsors to address prolonged trial timeline and high quality data. Rare Diseases and Precision Medicine Require Unique Approaches In Clinical...
BBCR embraces innovative strategy consulting for effective clinical development plan and regulatory strategy.
Our industry is looking for an innovative process. Focus must go to cost containment and value-based developments that allow sponsors to move more treatments to market faster. BBCR’s team expertise in rare diseases and precision medicine combined with SCIO approach...
PKAN drug fails Phase III trial: How to avoid a similar disaster.
Retrophin's experimental PKAN drug fosmetpantotenate fails phase III trial Pantothenate kinase-associated neurodegeneration (PKAN) is a rare disease characterized by a progressive neurodegenerative disorder and buildup of iron in the brain which is estimated to affect...
Inflammation is a recognized cause of Neurodegeneration.
Neuroinflammation is inflammation of the nervous tissue. It may be initiated in response to a variety of cues, including infection, traumatic brain injury, toxic metabolites, or autoimmunity. In the CNS, including the brain and spinal cord, microglia are the resident...
High cost and cycle time delays of protocol amendments
In practice, for a given clinical trial, it is not uncommon to have 3–5 protocol amendments after the initiation of the clinical trial. One of the major impacts of many protocol amendments is that the target patient population may have been shifted during the process,...
Potential of Gene Therapies in Rare Disorders
BBCR Consulting, for the first time this year, decided to attend on October 15th - 17th in London- UK, the European edition of the Gene Therapies in Rare Disorders conference. In our opinion, the event, as promised, proven to be: ”…uniquely focused conference that...
Generic EpiPen to reduce costs
Teva received approval to market generic versions of Mylan’s epinephrine auto-injector for emergency treatment of allergic reactions for both adults and children in August 2018 after several years of delays in getting the generics approved. Teva Pharmaceutical EpiPen...
Biologic treatments show promise in providing clinical solutions to a variety of diseases. Boston Biotech Clinical Research has experience in biologics for rare diseases and can help develop a targeted strategy for your needs.
Biologic treatments show promise in providing clinical solutions to a variety of diseases Due to biological’s unique characteristics, study designers may find that results in pre-clinical trials do not predict acute, chronic and late onset immunogenicity and safety....
Repurposing acts to lower the need for early stage clinical trials. BBCR’s team of industry experts can help match treatments to rare genetic conditions and unsolved diseases, then work with a product developer on the best plan to market.
People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. One attractive option of Drug Repurposing is to use a scientific approach to identify new uses for existing drugs. About a third of...
The Strategic Clinical Innovation Organization (SCIO) concept developed by BBCR was designed specifically to help pharmaceutical innovators address the concerns and maneuver around evolving challenges. SCIO allows for time and cost efficiencies, and risk mitigation.
Find opportunity for efficiencies early in the clinical development process A Clear Path to Approval The Strategic Clinical Innovation Organization (SCIO) concept developed by BBCR was designed specifically to help pharmaceutical innovators address the concerns and...
Collaborating with Boston Biotech Clinical Research can streamline the clinical trial process. We customize a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.
We invite you to read some of our Case Studies below PROJECT An executive at a pharmaceutical company asked BBCR to review its biosimilar pre-clinical data and prepare the IND package. In addition to evaluate the innovator approved indications to identify the fastest...
Biomarkers are now a routine part of drug development. BBCR can help you understand their role and move your product to market faster.
Biomarkers are now a routine part of drug development Identification and adoption of biomarkers are especially valuable in rare disease and precision medicine product development. The FDA recognizes biomarker development as a high priority area for future research....