
BBCR is dedicated to meeting the challenges of developers and investors seeking a clearer path to market. We invite you to bookmark our blog page and follow us across social media to keep abreast of timely and relevant topics in our industry.

BBCR is dedicated to meeting the challenges of developers and investors seeking a clearer path to market. We invite you to bookmark our blog page and follow us across social media to keep abreast of timely and relevant topics in our industry.

Our proprietary Strategic Clinical Innovation Organization (SCIO) concept was developed to address and maneuver around evolving challenges, allowing for time and cost efficiencies, and mitigating risk. BBCR works with clients in the areas of Biomarkers, Clinical Research consulting, Regulatory Affairs, and Early Clinical Development. To learn more about how BBCR can help with your research […]

Knowledge of disease and its Natural History is an essential element of any clinical development program. Real-World Data (RWD) is data relating to patient health status and/or the delivery of healthcare routinely collected from a variety of sources. Under the right conditions, data derived from real world sources can be used to build Rare diseases […]
We believe that understanding the perspective, challenges, and needs of our clients allows us to serve as a trusted adviser throughout the clinical development process. BBCR clients are domestic and international, small and medium sized drug and device biotechnology firms as well as venture capitalist. Clinical researchers and innovators come to us looking for the […]
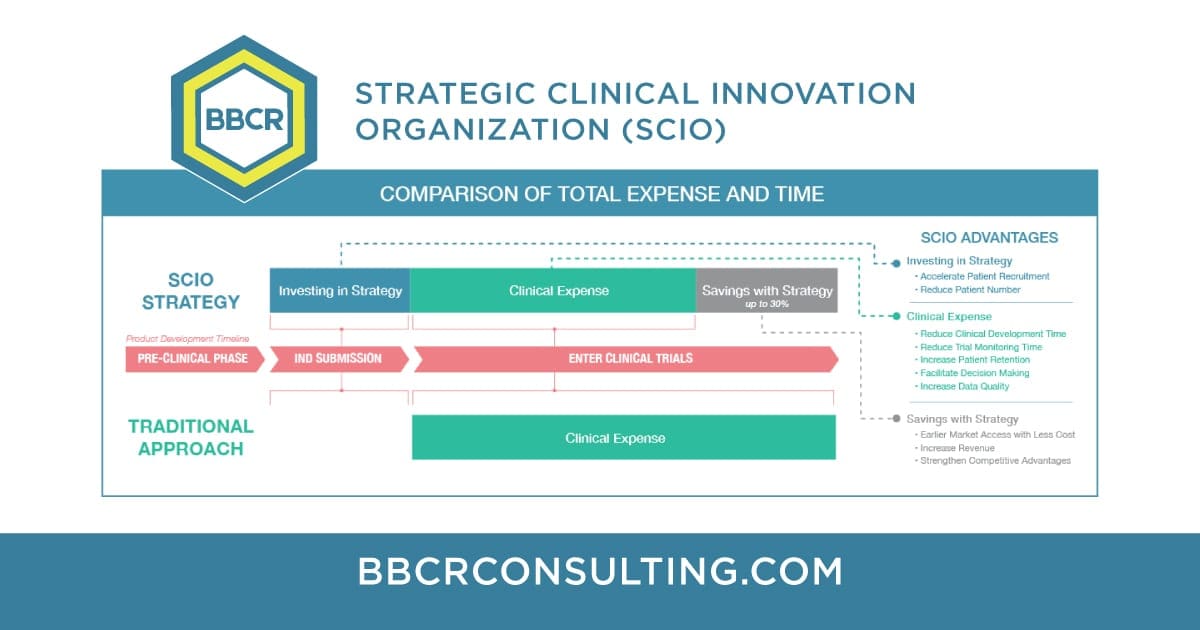
BBCR has developed an approach to meet the challenges of researchers and innovators seeking a clearer path to market. Flexible Road-Map Successful clinical development, for drug and medical device companies (small and large), requires an early strategy with a flexible road-map that makes it actionable and sustainable. Robust Clinical Data Customized Strategy and Road-Map allow […]

Depending on your project goals, our consultants work with you to design cost-effective early clinical studies that hold potential for reducing Phase III failures. Our services include: POM & POC Translational Research Clinical Plan & Study Design Phase 1 & 2 Protocol Development Medical Monitors Study Management

Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. BBCR Consulting offers world-class regulatory, clinical research, and biomarker consulting services that provide high-value, and support our clients’ operational and functional needs. Our process is designed […]

Expertise in integration of in vitro and in vivo analysis during early clinical research is a critical development milestone for efficient candidate development. In addition, the BBCR team guides in identifying the right target, the right biomarker, the right safety, the right patients and the right commercial potential. BBCR can assist clients across a variety […]
Biomarkers are considered a routine part of drug development. Learn more about how BBCR can help get your product to market more efficiently with proper plan development and strategy. Plan Development Insightful strategy is the most effective way to ensure quality when including biomarkers into your product development plan. Validation or Qualification Understanding where […]

Biomarkers are now a routine part of drug development The FDA recognizes biomarker development as a high priority area for future research. The FDA and European Medicines Agency (EMA) have developed similar processes for the qualification of biomarkers intended for use as companion diagnostics or for development and regulatory approval of a drug or therapeutic. […]