How we can help The BBCR team provides knowledge in cell, biologics and gene therapies BBCR addresses sponsors’ questions in the ever-changing regulatory environment We offer clinical strategy for orphan diseases and precision medicine RWE can be used to build orphan diseases evidence to support regulatory decisions BBCR serves small and medium sized Biotech Companies, […]
Strategy and TPP
The BBCR mission is to customize strategies, simplify clinical research, design cost-effective trials, streamline protocols, and create a regulatory roadmap. Learn more about our services by visiting bbcrconsulting.com
September 20th, 2022 | Strategy and TPPBBCR regularly partners with small and medium sized drug and device biotechnology firms. Our clients are innovators and clinical researchers looking for a more efficient path to approval, and come to us from across the globe. Reach out today to learn more.
September 15th, 2022 | Strategy and TPPThe BBCR mission is to simplify clinical research, encourage cost-effective trials, and help innovators navigate through the regulatory process. Small & Medium Sized Biotech Companies currently moving from pre-clinical studies toward clinical trials in need of interim Chief Medical Officer in need of regulatory strategy consultancy in need of preparing a pre-IND, orphan petition, IND, […]
BBCR specializes in strategy and early clinical research services from preclinical through Phase I and POC studies to enable informed, timely decision making for our clients.
August 31st, 2022 | Strategy and TPP
BBCR Consulting offers clinical, regulatory, translational, and biomarker consulting services that support our clients’ needs. Our process is designed to maximize time and cost efficiencies while mitigating risk. We partner with domestic and international companies. Our mission is to support pharma and biotech companies, and nurture their products’ strengths while improving efficiency and safety.
An attractive option of drug repurposing is to use a scientific approach to identify new uses for existing drugs thereby reducing the need for early stage clinical trials.
August 17th, 2022 | Strategy and TPP
People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. About a third of orphan approvals by the FDA since the program began have been mostly for repurposed mass-market drugs. BBCR’s team of industry experts can help match treatments to rare genetic conditions […]
BBCR offers expertise in the areas of Product Development Planning and Validation or Qualification when biomarkers are a component of your product development plan.
August 3rd, 2022 | Strategy and TPPWe are pleased to announce that BBCR Consulting President, Candida Fratazzi, MD will be the keynote speaker at the upcoming 9th Clinical Trials Strategic Summit happening in San Francisco on October 27th and 28th, 2022
July 29th, 2022 | Strategy and TPP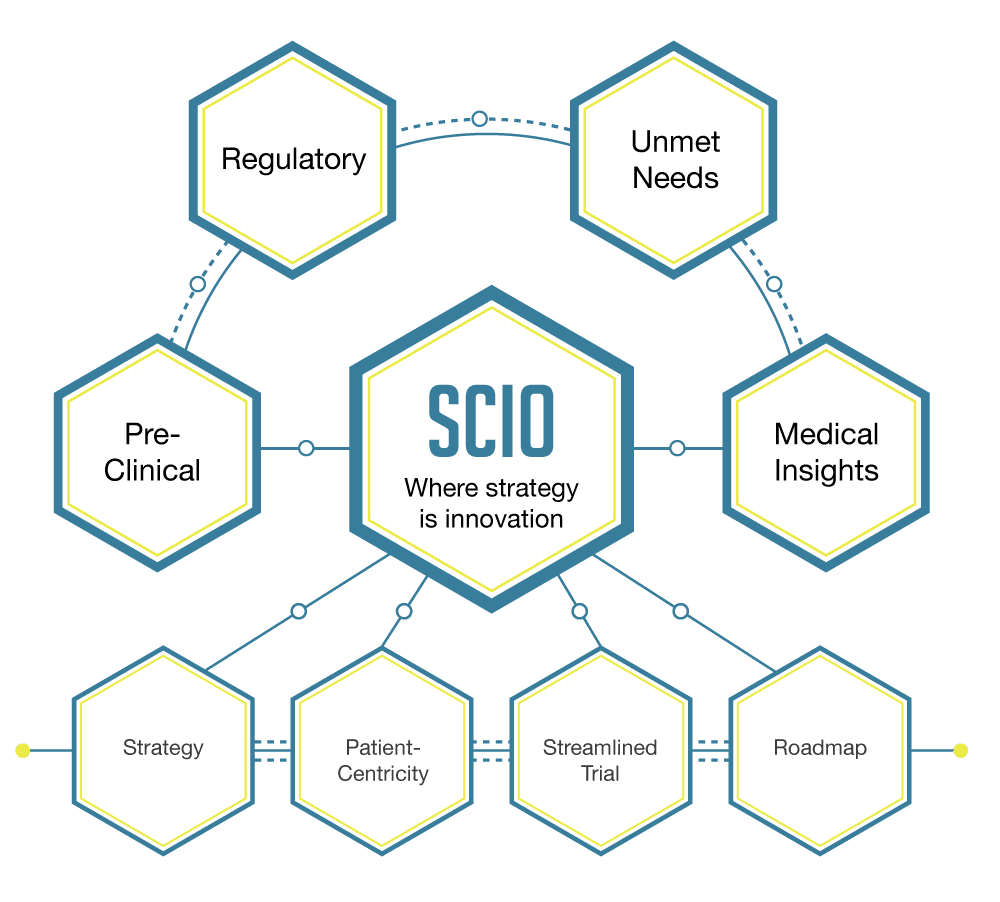
BBCR is looking forward to attending this important event and expresses gratitude to Agile Falcon Strategic Group LLC for the opportunity to be a part of the upcoming Summit. The 9th Clinical Trials Strategic Summit (CTSS) will be taking place in San Francisco October 27th and 28th, 2022. We hope to see you there!
Strategic Consulting and the SCIO Process – Paving the Way for Drug Development
July 19th, 2022 | Strategy and TPPSCIO is critical for accelerating approval and significantly reducing the costs of drug and medical device development. One of the major challenges facing this industry is the rising cost of clinical trials. Thus, it is crucial to ensure that trials are significantly more efficient and cost-effective with the involvement of strategy at the time of […]
Why choose BBCR for your product development needs?
July 12th, 2022 | Strategy and TPP
BBCR can help streamline product development and help to successfully navigate your clinical and regulatory journey. BBCR focuses on: Acceleration Cost-effectiveness Risk reduction Data collection and analysis support
BBCR has extensive experience in early development Phase 1 and 2, from pre-clinical to pre-IND. BBCR’s early drug development experience includes the development of clinical strategy and plan, including target product profiles and clinical protocols.
June 30th, 2022 | Strategy and TPP
Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process and strategy. Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements.
BBCR’s team of professionals can help with your Pre-ind Integrated Development Plan
June 15th, 2022 | Strategy and TPP
A strategy that clearly marks the path to success creates opportunity to reduce risk. BBCR works with clients to achieve a successful pre-IND meeting by: Reviewing scientific data Setting regulatory strategy Setting integrated plan Creating messages and outlines Developing realistic Q&As Providing constructive critique
