Biomarkers are now a routine part of drug development In rare diseases and precision medicine, implementation of biomarkers during product translation into clinic and early clinical development, moves treatment to market faster.

Biomarkers are now a routine part of drug development In rare diseases and precision medicine, implementation of biomarkers during product translation into clinic and early clinical development, moves treatment to market faster.

Given the global Covid19 pandemic and the global nature of the pharmaceutical and biotech industry, key operational areas have and will continue to be impacted. As with any emergency response, the key to management is implementing mitigation plans. Early stage pharmaceutical and biotech companies need to be proactive in developing contingency plans to jump start […]

Drug repurposing acts to lower the need for early stage clinical trials and can help identify new uses for existing drugs. People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. One attractive option of Drug Repurposing is to use a scientific approach […]

The BBCR team designs Proof of Concept (PoC) Trials and Proof of Mechanism (PoM) studies with the drug clinical plan and regulatory strategy in mind. Proof of Mechanism (PoM) Usually in Healthy Volunteers, Phase 1 study Essential for the selection of appropriate dose for PoC, disease model and biomarkers Investigate drug concentration at the target […]
From CAR T to CAR macrophage: The improvement of CAR cell therapy in solid cancer treatment The Chimeric Antigen Receptor T (CAR T) cell technology is a revolutionary therapy and has shown promising clinical response in cancer treatment. In 2017, anti-CD19 CAR T cell therapy against B cell malignancies was approved by US FDA. However, […]
Obesity-induced gut microbiome composition is linked with graft-versus-host disease (GVHD) in mice and human after allogeneic hematopoietic stem cell transplantation (HSCT) By: Dr. Maria Niu GVHD is a potentially severe immune disorder related to HSCT. In brief, the donor T cells recognize the recipient as non-self or foreign, therefore trigger a wide range of immune […]
Gene Therapy improved vision in patients with leber hereditary optic neuropathy (LHON) By: Dr. Maria Niu Mitochondria, powerhouses of eukaryotic cells, is a certain kind of cytoplasmic organelle and plays a critical role in energy production. Mitochondria dysfunction results in a broad spectrum of multisystem disorders. LHON is a mitochondrial neurodegenerative disease typically caused by […]
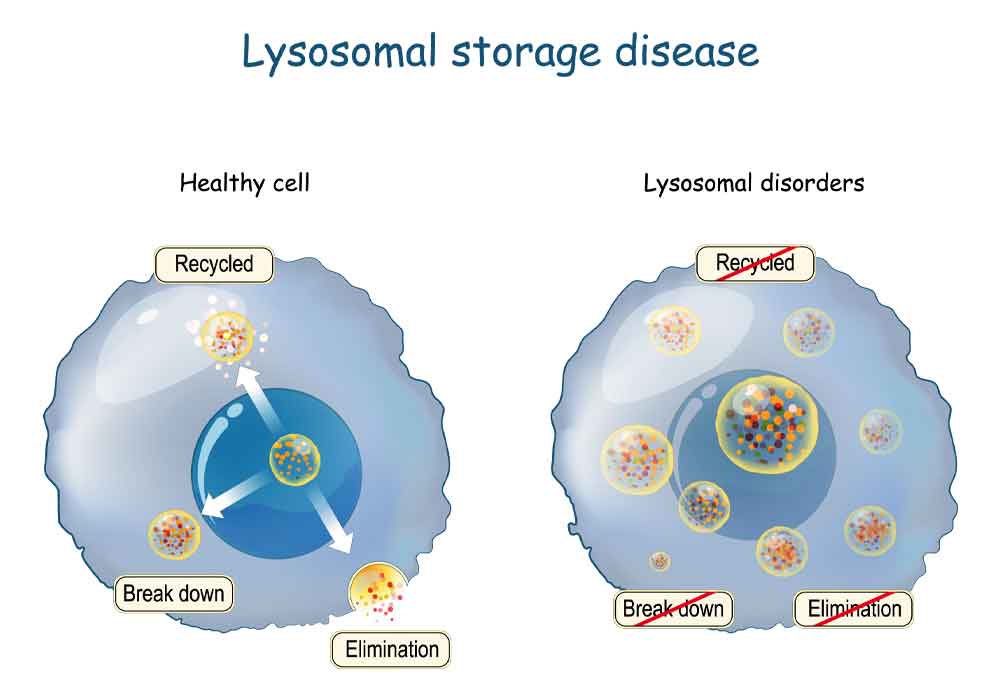
Emerging cell and gene therapy may offer sustained long-term correction for LSD patients Dr. Maria Niu Lysosomal storage diseases (LSDs) are rare inherited metabolic diseases and characterized by the accumulation of substrates in excess in various organs’ cells due to lysosomes’ defective functioning. The combined incidence of LSDs is between 1 in 5000 to 1 […]

Biologic treatments show promise in providing clinical solutions to a variety of diseases including rare cancers and precision medicine. Services include: Indications analysis and prioritization Strategic drug assessment Clinical study design and protocol Biomarker strategy Early Clinical Development FDA meeting and submission Pre-ND integrated development plan CRO and project management Study remediation and rescue […]

Expertise in integration of in vitro and in vivo analysis during early clinical research is a critical development milestone for efficient candidate development. In addition, the BBCR team guides in identifying the right target, the right biomarker, the right safety, the right patients and the right commercial potential. BBCR can assist clients across a variety […]