Real-world evidence (RWE) is increasingly important in clinical trial design and drug approval. RWE is clinical evidence related to patient health status and treatment safety and efficacy that are generated in clinical practice. The FDA developed a framework to use RWE, which may combine with orphan, fast track, breakthrough therapy designation, and accelerated approval to […]
Drug Development
BBCR’s team of experts is highly experienced in the areas of Real World Evidence (RWE) for Control Arm, Post-approval Requirements and Disease Natural History. Reach out today to learn more.
September 8th, 2022 | Drug DevelopmentAn attractive option of drug repurposing is to use a scientific approach to identify new uses for existing drugs thereby reducing the need for early stage clinical trials.
August 17th, 2022 | Drug Development
People tend to believe that a repurposed therapy can never be truly novel or transformative. Nothing could be further from the truth. About a third of orphan approvals by the FDA since the program began have been mostly for repurposed mass-market drugs. BBCR’s team of industry experts can help match treatments to rare genetic conditions […]
BBCR meets clients’ needs in the fast paced and ever-changing regulatory environment. As specialists in Orphan and Personalized Medicine, BBCR helps clients identify areas of need or economic interest and helps them find homes for treatments for rare diseases and precision medicine.
August 10th, 2022 | Drug Development
BBCR helps orphan drug developers find direction in clinical trials involving biologics, biosimilars, small molecules, medical devices, and repurposing. BBCR consultants have the experience to guide you through the development process with a clinical plan and a regulatory strategy. Our team will empower you with medical insight, effective regulatory expertise, strategy, and streamlined early clinical […]
Strategic Consulting and the SCIO Process – Paving the Way for Drug Development
July 19th, 2022 | Drug DevelopmentSCIO is critical for accelerating approval and significantly reducing the costs of drug and medical device development. One of the major challenges facing this industry is the rising cost of clinical trials. Thus, it is crucial to ensure that trials are significantly more efficient and cost-effective with the involvement of strategy at the time of […]
BBCR Consulting offers world-class regulatory, clinical research, and biomarker consulting services that provide high-value, and support our clients’ operational and functional needs. Our SCIO process is designed to maximize time efficiencies, risk mitigation, and cost savings.
May 31st, 2022 | Drug Development
Our experienced team helps each client reach their specific goals by customizing a clinical and regulatory road map of simplified programs and streamlined protocols to meet our clients’ requirements. BBCR Consulting has created a process called SCIO (Strategic Clinical Innovation Organization), designed to change the path from pre-clinical trial to market by creating a regulatory […]
Streamlining clinical development with clinical and regulatory expertise is the cornerstone of our business. BBCR’s mission is to streamline your clinical development while reducing costs and improving safety.
May 24th, 2022 | Drug Development
BBCR Consulting offers world-class regulatory, clinical research, and biomarker consulting services that provide high-value, and support our clients’ operational and functional needs. Our process is designed to maximize time efficiencies, risk mitigation, and cost savings. Innovating the process of drug development with clinical and regulatory expertise is the central value of our business. BBCR’s mission […]
BBCR embraces innovative strategy consulting for highly effective clinical development plan and regulatory strategy. Reach out today to learn more about how we can help advance your project.
May 18th, 2022 | Drug Development
Our industry needs innovative strategies, and reduced-risk clinical trials. BBCR clinical development services and drug development consulting integrates real world evidence into clinical development plans and regulatory strategies. We ensure that focus goes towards cost containment and value-based developments that allow sponsors to move more treatments to market faster. BBCR’s team expertise in rare diseases […]
SCIO helps early stage biotech company evaluation. Learn why adopting this clinical roadmap and development strategy is critical to your success in bringing your product to market.
April 20th, 2022 | Drug Development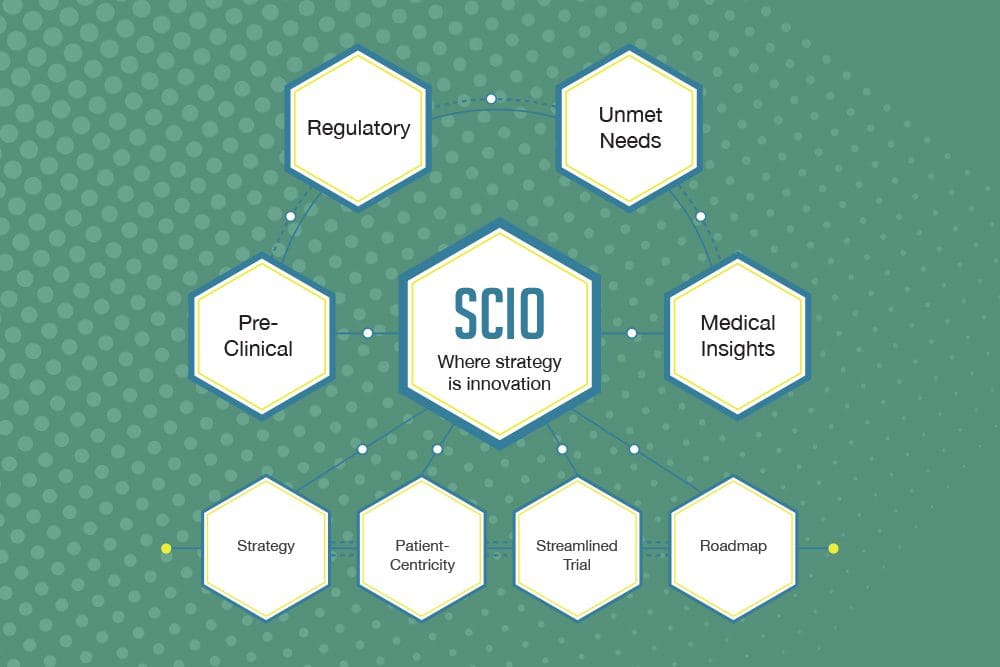
How can SCIO help? To enhance your position, it becomes crucial to demonstrate that your product/technology is the “safe” option for investment and a stand-out opportunity by including a clinical roadmap and development strategy of your product/technology. This roadmap illuminates clearly for the investment team what the target indications will be and the paths to […]
BBCR’s experience and understanding of how the FDA views orphan applications and structuring a development program to deliver the data and rationale to satisfy the FDA can substantially reduce the review period and increase approval.
February 16th, 2022 | Drug Development
A strategic approach creates opportunity for time efficiencies The Orphan Drug Act is an important piece of legislation that uses financial incentives to encourage the development of drugs that treat rare diseases and precision medicine impacting disease sub-populations. BBCR’s team of experts can help your company create a roadmap specially customized to ensure successful product […]
Drug Repurposing can lower the need for early stage clinical trials. BBCR’s team of industry experts can help match treatments to rare genetic conditions and unsolved diseases.
December 9th, 2021 | Drug Development
According to recently released data, 12 out of the 28 drugs approved in the first quarter of 2020 by the FDA were repurposed drugs. The drug repurposing approval process is an effective tool that accelerates the development of drugs for new indications or drug reformulation. In addition, the development of drug repurposing compared to a […]
