Strategies for Orphan Diseases
“The beginning is the most important part of the work.” — PlatoWelcome To BBCR
Providing Expert Guidance for Orphan Drug Development
BBCR is dedicated to supporting pharmaceutical innovators in the specialized rare diseases and orphan drug indications by developing and nurturing the product’s unique strengths.
Our operational mission is to craft customized strategies that achieve cost-effective trials by 1) simplifying clinical plans, 2) streamlining trial protocols and 3) creating robust regulatory roadmaps for speed to market.
THE BBCR TEAM PROVIDEs CONSULTANCY IN CELL, BIOLOGICS, AND GENE THERAPIES

BBCR ADDRESSES SPONSORS' QUESTIONS IN THE EVER-CHANGING REGULATORY ENVIRONMENT
CLINICAL STRATEGY FOR ORPHAN DISEASES AND PRECISION MEDICINE
The BBCR team is experienced in building evidence of orphan disease using RWE to support regulatory decisions.
BBCR serves small and medium-sized biotech and medical devices companies, venture capitalists, and investors
A Clear Path to Approval
Strategic Clinical Innovation Organization
Who We Serve
Small & Medium Sized Biotech and Device Companies
- Moving from pre-clinical studies to formal clinical studies
- Crafting the clinical development plan
- Building the study synopsis and/protocols
- Looking for an interim Chief Medical Officer function
- Searching for a study medical monitor
- Requiring a regulatory strategy consultancy
- Preparing a pre-IND, Orphan petition or IND package
- Needing assistance in CRO selection and Management
- Evaluating potential trial remediation and rescue
Venture Capitalists/Investors
- Due diligence and ongoing monitoring of regulatory, safety and efficacy aspects of project/portfolio progress
Therapeutic Areas
- Orphan Diseases
- Immuno-oncology
- Neurology/CNS
- Cell and Gene Therapy
- Repurposing Drugs
- More Therapy Areas
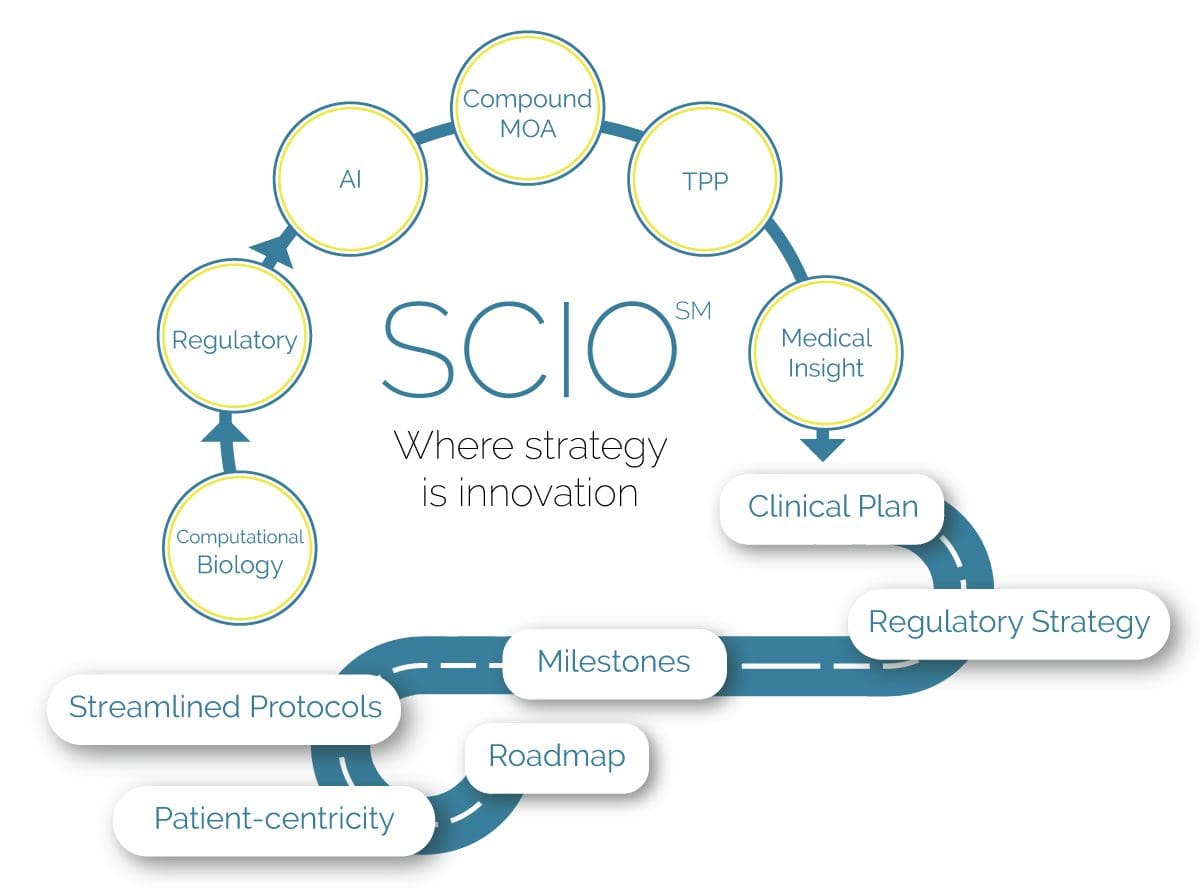
Our Strategic Clinical Innovation Organization (SCIO SM) method enables our clients to save time, create cost efficiencies, and reduce risk during the process of achieving optimal product market positioning.
THE SCIO SM METHOD

Customized Strategies for Streamlined Clinical Development
Specializing in rare diseases, Boston Biotech Clinical Research works with biotech, pharmaceutical, device companies and investors to streamline the clinical trial process.
Our experienced team helps clients reach their goals by customizing a clinical and regulatory roadmap of simplified programs and streamlined protocols.
The Innovative Pathway to Approval
def. 1.
For clinical trials, cost efficiency, and risk management in clinical trials from pre-clinical to market.
def. 2.
To develop product-specific clinical and regulatory strategies as part of a pre-IND, IND or Orphan petition and before tactical clinical activities (i.e. CRO) start.
[Latin SCIO – to know, – to understand, – know how to]

Why SCIO SM?
The SCIO SM method is the solution for cost-effectiveness and risk management in clinical development.

“Integrity, excellence, and high appreciation of project’s needs as well as strategic solutions that are innovative and sustainable.”
Years of Combined Experience
Published Studies
Developed Products
Welcome to BBCR Voice

BBCR partners with clients to identify, develop, and advance a product’s unique strengths from early concept through to market. Our consultancy is grounded in deep expertise across cell, biologic, and gene therapies, with a particular focus on rare disease.
BBCR partners with sponsors and investors to navigate complex paths to market, strengthening product potential while advancing efficiency and safety. Through strategic expertise and early clinical research services, we support informed, timely decisions at every stage...





