POM and POC
Designing the Proof of Concept (PoC) and Proof of Mechanism (PoM) studies with the clinical plan and regulatory strategy in mind
Proof of Mechanism (PoM)
- Usually in Healthy Volunteers, Phase 1 study if Phase1/2 in patients
- Essential for the selection of appropriate dose for PoC, disease model, and biomarkers
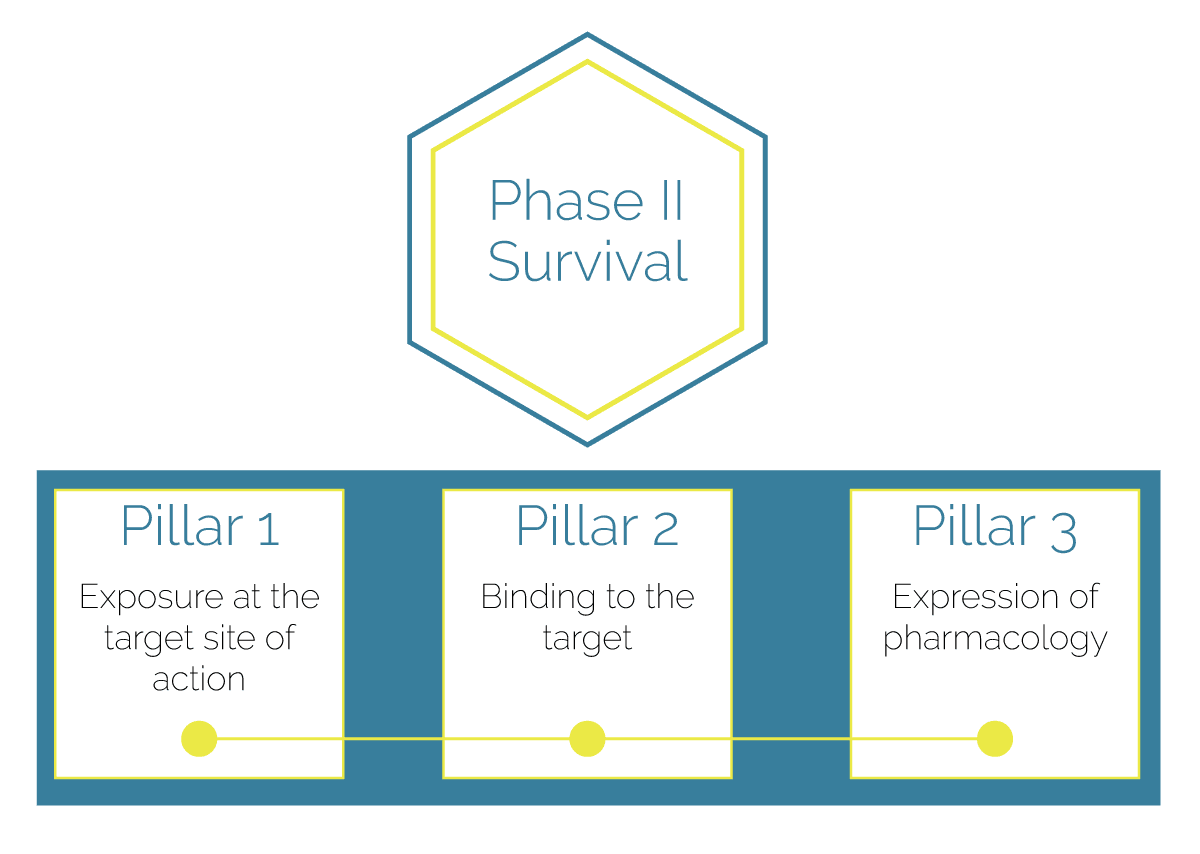
- Investigate drug concentration at the target site of action
- Investigate drug engagement with target molecular receptor or enzyme (e.g., Receptor binding)
- Investigate if the biological response matches the expected effect
- Analyze PK/PD relationship
Proof of Mechanism (PoC)
- Phase 2 study in patients with the selected disease
- Inform on indicative effectiveness and desired clinical activity
- PoC lacks statistical power
- High-quality PoC study yield insight into drug interaction with disease physiology
- High-quality reads-out enable highly educated Go/noGo decisions
The PoM and PoC trials’ design is based on the Pillar Theorem: