Accelerated Orphan Drug Approval: Surrogate Endpoints
Candida Fratazzi * and Jixiao Niu
World Journal of Advanced Pharmaceutical and Medical Research, 2022, 02(01), 001–007
BBCR Consulting, One Broadway, Cambridge, MA, USA
Abstract
Today, orphan drug development is confronted with significant challenges represented by the considerable complexity, diversity of clinical manifestations, and competition in study recruitment. Thus, surrogate endpoints adoption plays a crucial role in rare disease trials by minimizing costs, the number of subjects, and study duration. Surrogate endpoints, to substitute for a direct measure of how patients feel, function, or survive, must be biomarkers that directly correlate with disease clinical manifestations and predict the impact of study drug on the long-term disease progression. Validation of surrogate endpoints for accuracy and sensitivity is essential to maximize its benefit and utility. These validation criteria include reliability, reproducibility, keenness, and a direct reflection of patient feeling, function, or survival upon treatment.
On the other hand, the selection of surrogate endpoints may be pretty complex, and making mistakes may lead to inaccurate estimates of the clinical benefit. Finally, surrogate endpoints contribute to a composite endpoint when studying drug benefits patients in multiple ways, and not all the measured components are detected in each patient. Furthermore, a composite endpoint comprised of multiple surrogate endpoints could improve statistical efficiency. Selection and qualification of biomarkers for surrogate endpoints and accelerated market approval is often a complex process that requires experience and method. Today, we contributed to developing orphan drugs for many rare diseases, including Neurological Diseases, Lysosomal Storage Diseases, Metabolic Conditions, Immune Disorders, and Cancers. In conclusion, surrogate endpoints play an essential role in orphan drug development, benefiting patients and the healthcare system.
Keywords: Biomarkers; Surrogate endpoints; Orphan drugs; Accelerated market approval; Strategic Clinical Innovation Organization; Rare diseases
1. Introduction
Rare Diseases are conditions that affect less than 200,000 people in the U.S. However, over 7,000 Rare Diseases affect more than 400,000,000 people worldwide, including ~25 million in the U.S. and ~30 million in Europe [1]. In recent years, the number of orphan drugs approved has increased significantly. However, developing and marketing orphan drugs remains a significant challenge with a substantial stumbling block represented by the considerable complexity and variety of clinical manifestations in rare diseases. The Food and Drug Administration (FDA) guidelines highlight the benefits of orphan drug market approval [2]. Today, the surrogate endpoints adoption in orphan drug trials overcomes some of the burdens and challenges, minimize trial costs, reduce the number of subjects in clinical trials and the study duration. Thus, surrogate endpoints play a crucial role in orphan drug development [3].
2. What is a Biomarker?
Biomarkers are classified into different categories, which, based on functions, are diagnostic, prognostic, predictive, and safety. Biomarkers are objective medical signs (as opposed to symptoms reported by the patient) used to measure the presence or progress, or treatment effects on the disease. A biomarker is a defining characteristic that indicates normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions [4]. These are four types of biomarkers which, based on the characteristics, can be molecular (i.e., blood glucose), histologic (i.e., tumor type), radiographic (i.e.tumor, size), or physiological (i.e., blood pressure).
3. What is a Surrogate Endpoint?
A surrogate endpoint is defined as a biomarker intended to substitute the clinical endpoint. Endpoints are measurable outcomes to assess the product’s benefit and address the objectives of a clinical trial. The FDA defines a surrogate endpoint as an endpoint to substitute for a direct measure of how a patient feels, functions, or survives [2]. The majority of surrogate endpoints are biomarkers covering clinical endpoints. These biomarkers must have the defining characteristics of being an objective indicator of a normal biological process, pathogenic process, or biological response to therapeutic intervention [4]. However, a clinical endpoint could also be a surrogate endpoint when an intermediate clinical endpoint replaces the Endpoint of interest [5] (Fig1).
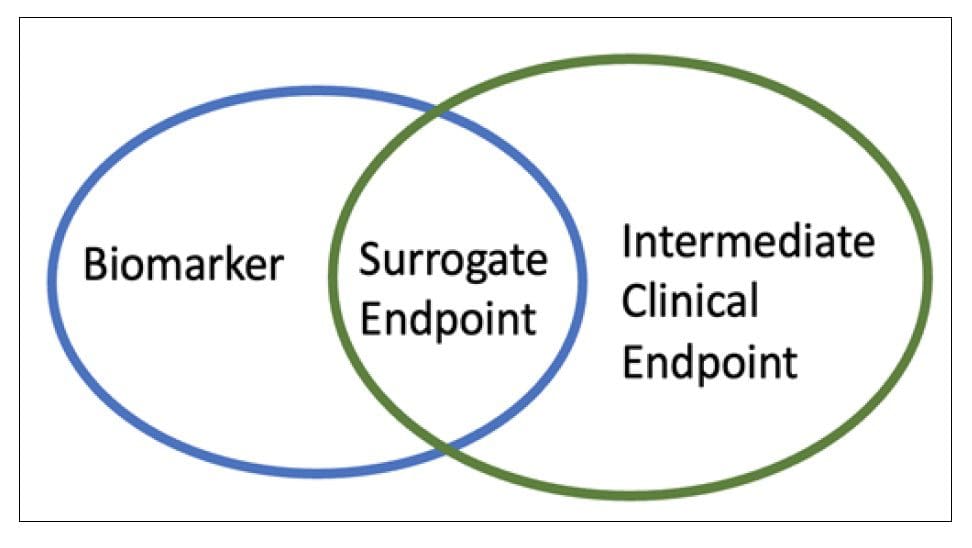
Figure 1 Assessments in clinical trials. Surrogate endpoint could be a biomarkers or an intermediate clinical endpoints
Surrogate endpoints have been widely applied in orphan drugs to address the challenges caused by the wide range of clinical manifestations, severity, and long-term disease progression of most orphan diseases. To best prove a clinical benefit, the surrogate endpoint should be measurable and verifiable, and it should predict the clinical endpoint rather than measuring the clinical manifestation. Several surrogate endpoints include laboratory measurement, radiographic image, physical sign, etc. [6]. The FDA has released a table summarizing adult and pediatric surrogate endpoints adopted to approve small molecules and biological drugs. About ~20% (24/121) of surrogate endpoints, shown in the table, belong to developing treatments for adults with rare diseases [7]. This list of approved surrogate endpoints also includes non-orphan diseases such as cancer and diabetes.
4. Advantages and Disadvantages of Surrogate Endpoints in Rare Diseases
Surrogate endpoints have shown distinct advantages/disadvantages (Table1).
4.1. Advantages
Surrogate endpoints shorten clinical trials duration. Measuring the clinical outcome is often a time-consuming procedure, especially in Rare Diseases. The time to measure biomarkers changes is shorter than assessing clinical parameters changes. Thus, surrogate endpoints adoption allow for much faster assessment and evaluation of the study drug treatment benefit on disease progression. Ultimately, a surrogate endpoint shortens the clinical trial duration and accelerates the NDA submission [8]. Moreover, adopting a surrogate endpoint reduces the number of subjects required to establish the statistical efficacy significance in clinical trials. The laboratory parameters are usually quantitative and unbiased compared to clinical parameters that are easily influenced by variable severity and difference of clinical manifestations [9].
Furthermore, surrogate endpoints are objective and standardized measurements unraveling the underlying mechanisms of diseases [10].
4.2. Disadvantages
The significant benefit of using surrogate endpoints includes shorter study time, fewer subjects, no bias, and a reduced trial cost. However, we cannot undermine the risk of using surrogate endpoints. Table 1 summarizes those risks well. In the past, a few surrogate endpoints proved to be inaccurate. Thus, it is mandatory that a surrogate endpoint is reliable in predicting the clinical benefit linked to it. Biomarkers have been used as surrogate endpoints in a wide range of diseases. However, every biomarker to qualify as a surrogate endpoint must demonstrate an ability to predict the outcome of the related clinical manifestation and the treatment effect. Biomarkers have to be intrinsically related to the pathogenesis of the disease, so if the hypothesis of disorders mechanism is incorrect, the biomarkers may lead to inaccurate estimates of the clinical benefit [11].
Table 1 Advantages and Disadvantages of Surrogate Endpoints
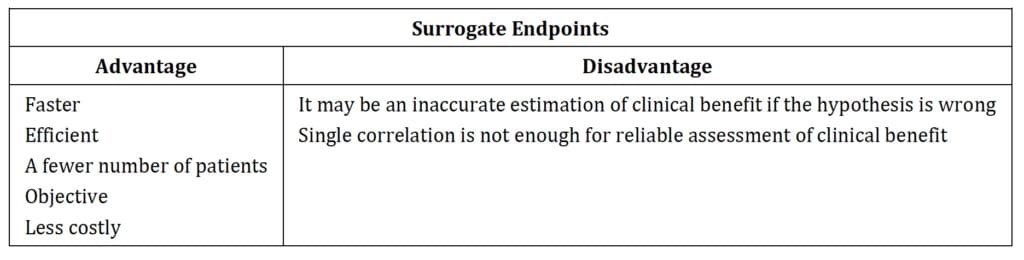
Moreover, some biomarkers may not be the significant factor affecting the change toward the progress of the disease and the tested clinical benefit. Using only one biomarker as a surrogate endpoint may not detect the clinical benefit of interest if multiple factors are involved in the pathogenesis and progression of the disease [12, 13].
5. Case Study
In Duchenne Muscular Dystrophy (DMD) trials, the six-minute walk test (6MWT) is the most commonly adopted surrogate endpoint. However, this measure is subjective to the pediatric patient’s feelings which may heavily influence the test results. Thus, the 6MWT is not reliable to measure the effect of a treatment, at every time point, during the clinical trial.
The dystrophin measurement represents the underlying cause of DMD and directly reflects the muscle function and disease progression, thus providing a more objective and more accurate indication of clinical benefit [11].
Unfortunately, the expression of skeletal muscular dystrophin, which is more reliable in detecting the disease progression, cannot be easily tested because it requires a muscle biopsy and other technical reasons. Indeed, precise assessment of dystrophin expression is also challenging due to the protein’s low abundance and its large size. The current techniques include Western blot, mass spectrometry, and ELISA, which measure dystrophin in whole tissue samples instead of fiber-by-fiber. In addition, some techniques are limited by quantification analysis. For example, the lower limit of quantification (LLOQ) of 3-5% for mass is too high in DMD patients [14].
6. What does a Validation of Surrogate Endpoints include?
Validation is the process of assessing the biomarker and its measurement performance characteristics and determining the range of conditions under which the biomarker will give reproducible and accurate results.
Extensive evidence gathering from mechanistic studies, clinical trials, or standard of care reviews is required to validate a surrogate endpoint. That evidence must show that the surrogate endpoint accurately predicts the clinical benefit intended by the therapeutic intervention. In other words, those validated surrogate endpoints should be strongly supported by mechanistic rationale and clinical data
[4]. In general, surrogate endpoints validation must meet several stringent validation criteria to be approved as a surrogate endpoint.
6.1. Reliable and Reproducible measure
The surrogate endpoints provide reliable evidence of clinical benefit. The surrogate endpoint should be a trustworthy measure that accurately reflects the clinical response upon intervention. Besides, the selected surrogate endpoint should reproducibly evaluate the benefits and risks of an intervention [15, 16]. The reproducibility facilitates comparisons among different arms in clinical studies.
For example, Chest computed tomography (C.T.) is used as a surrogate endpoint in the clinical trial of cystic fibrosis lung disease because chest C.T. quantitatively evaluates the structural abnormalities [17], and it is reproducible.
6.2. Sensitive to the effect of the intervention
The surrogate endpoint should fully predict the improvement of clinical benefit upon intervention. It is an indicator of routine physical or pathologic processes and a sensitive marker of meaningful changes during the disease progression. The sensitivity of the surrogate endpoint plays a vital role in reducing the study size and duration. The sensitivity of the surrogate endpoint also increases the statistical power of the data comparing the difference between the study drug and the control arms [18].
6.3. Directly reflected the disease pathway and correlated with patients
The appropriate approach to surrogate endpoint validation is to determine whether it closely correlates with the causal pathway. A surrogate endpoint may be misused if intended to provide clinical efficacy relying on an outcome unlikely linked to the disease mechanisms [12]. In addition, the surrogate endpoint must give evidence on the clinical benefit of the study drug for the patients. In other words, the endpoint should directly measure the patient feeling, function, or survival upon treatment.
The framework for validating the surrogate endpoint in Lysosomal acid lipase (LAL) deficiency is illustrated below.
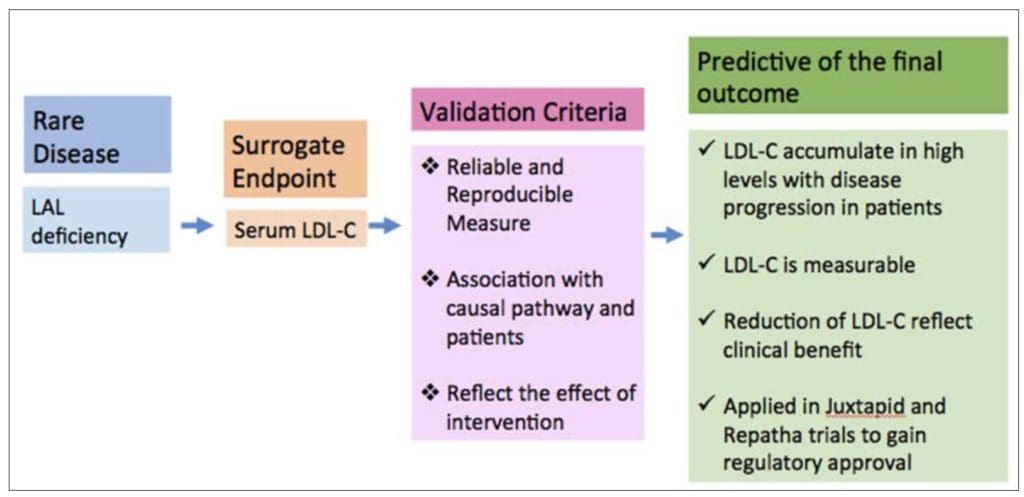
Serum LDL-C met the validation criteria and was adopted as a surrogate endpoint. Abbreviation: LAL, Lysosomial acid lipase, LDL-C, Low density lipoprotein-cholesterol
Figure 2 Framework of Validation of Surrogate Endpoint
7. Surrogate Endpoints in Oncology Trials
Surrogate endpoints are also widely applied in oncology trials. Between 2008 and 2017, the FDA has approved 135 drugs for rare cancers, of which 101 (75%) were also approved by the EMA. EMA granted orphan designation to 41/101 (41%), of which 9 were biomarker-derived drugs [19].
Between 2009 and 2014, the FDA-approved drugs for 83 oncological indications: 55 (66%) were approved on surrogate endpoints, of which 31 approved on response rate and 24 on progression-free survival (PFS) [20]. Twenty-five out of these 55 approvals were accelerated.
In oncology, no perfect surrogate does exist that can predict with reasonable accuracy the endpoint of interest. Thus, the FDA grants accelerated approval when a surrogate benefit shows’ likely likelihood to predict’ true clinical efficacy in survival or quality of life (QoL). While formal approvals are granted when a drug demonstrates benefit in ‘established’ surrogate endpoints [20]. Tumor assessment surrogate endpoints are the most commonly used. These endpoints include Disease-free survival, Objective response rate, Complete response, and time to progression / progression-free survival. Disease-free survival is frequently used after surgery and when there is a complete response. The objective response rate is assessed with the RECIST criteria, which standardize tumor shrinkage evaluation. Complete response is commonly used in leukemia, lymphoma, and myeloma. In addition, it is essential to evaluate symptoms related to disease or adverse events that are captured in QoL assessments [21].
7.1. Composite Endpoint to combine multiple Surrogate Endpoints
The composite endpoint is an endpoint resulting from the combination of at least two or more individual endpoints. The composite endpoint is especially useful in Rare Diseases where low event rates and small patients’ size are often the rules. Composite endpoints are an advantage when dealing with a drug that can benefit patients in multiple ways. Occasionally, the intervention may affect one component only rather than several components. In that case, a composite endpoint would diminish the likelihood of a statistically significant difference [21]. A composite endpoint could be a calculation score based on multiple components, thus providing a complete characterization of the intervention. Accordingly, surrogate endpoints involving biomarkers can be components of a composite endpoint [22]. In other words, multiple surrogate and clinical assessments can contribute to a composite endpoint. A composite endpoint detects statistical significance even if not all the measured components are seen in each patient.
Conversely, a composite endpoint is suitable when the study drug only affects one parameter of the measurements, part of the composite endpoint. Composite endpoints as surrogate endpoints should be implemented, when appropriate, to reduce trial patients’ size, decrease development costs, and increase statistical efficiency [23]. Therefore, both advantages and limitations should be carefully considered for selecting either composite and surrogate endpoints.
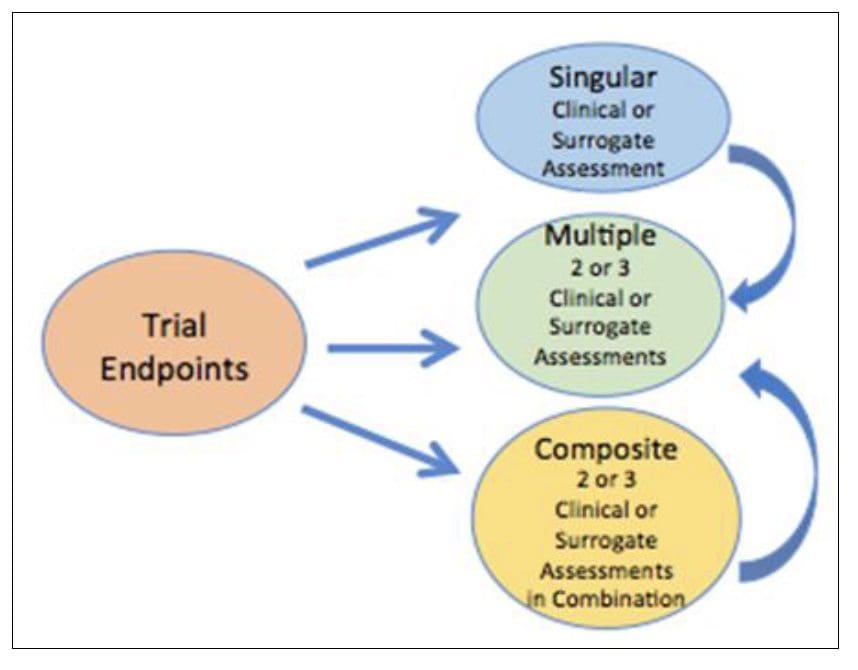
Figure 3 Endpoints composition in clinical trials There are three major types of trial endpoints including singular and clinical or surrogate assessment and/or composite endpoint
8. Conclusion
Our interest in surrogate endpoints and biomarkers adoption in orphan drug development rises from the opportunity to reduce cost and risk and streamline clinical trials in orphan diseases. Surrogate endpoints have proven to overcome clinical trials burdens and challenges that significantly impact trial cost and study duration. The FDA defines the surrogate endpoint as a trial endpoint that directly measures how a patient feels, functions, or survives. Selecting the suitable surrogate endpoint remains critical. The selected biomarkers must directly correlate with the clinical manifestation and predict the impact of the study drug on the long-term disease progression. Adoption of surrogate endpoints offers many advantages, including shorter study time, fewer study subjects, no bias, and saving cost.
On the other hand, we should not underestimate the possible disadvantages of a primary surrogate endpoint. If the biomarker selected is unrelated to the disease’s mechanism, it may result in inaccurate estimates of the clinical benefit. The DMD case study is presented to suggest the complexity of the surrogate endpoint selection.
Validation is required for a biomarker to be approved as a surrogate endpoint. Biomarkers of surrogate endpoint must be reliable, reproducible, and sensitive in reflecting the clinical benefits for the patients. Surrogate endpoints are also widely applied to rare cancer trials even if, up to date, no reliable surrogates are available that predict the endpoint of interest.
Finally, surrogate endpoints contribute to a composite endpoint when studying drug benefits patients in multiple ways, and not all the measured components are detected in each patient. The selection of appropriately validated surrogate endpoints substantially promotes orphan drug development and speeds up the regulatory approval of innovative technologies which benefit the patients and support the healthcare systems.
Selection and qualification of biomarkers for surrogate endpoints and accelerated market approval is often a complex process that requires experience and method. The Strategic Clinical Innovation Organization SM (SCIO) is an early clinical development process that addresses complex multifunctional research.
BBCR Consulting has a long experience in orphan drug regulatory strategy, surrogate endpoint selection, biomarker validation, and clinical plan. Today, we contributed to developing orphan drugs for many rare diseases, including Neurological Diseases, Lysosomal Storage Diseases, Metabolic Conditions, Immune Disorders, and Cancers. We are dedicated to continuing our contribution to orphan drug development.
Compliance with ethical standards
Acknowledgments
Acknowledgments to Claudio Carini M.D., Ph.D., FRCPath for reviewing the manuscript.
Disclosure of conflict of interest
The authors disclose no potential conflicts of interest.
References
[1] Nguengang Wakap, S., et al., Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet, 2020. 28(2): p. 165-173.
[2] The US. FDA. Surrogate endpoint resources for drug and biologic development [Internet]. July 24, 2018. Available from https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resources-drug-and-biologic-development.
[3] Ciani, O., et al., Use of surrogate end points in healthcare policy: a proposal for adoption of a validation framework. Nat Rev Drug Discov, 2016. 15(7): p. 516.
[4] in BEST (Biomarkers, EndpointS, and other Tools) Resource. 2016: Silver Spring (MD).
[5] European Network for Health Technology Assessment. Guideline: Endpoints used in relative effectiveness assessment of pharmaceuticals Surrogate Endpoints. February 2013. Available from https://www.eunethta.eu/wp-content/uploads/2018/01/Endpoints-used-in-Relative-Effectiveness-Assessment-Surrogate-Endpoints_Amended-JA1-Guideline_Final-Nov-2015.pdf.
[6] Definitive Healthcare. Available from https://www.definitivehc.com/resources/glossary/surrogate-endpoint.
[7] The US. FDA. Table of surrogate endpoints that were the basis of drug approval or licensure. 16 September 2021. Available from https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure.
[8] Katz, R., Biomarkers and surrogate markers: an FDA perspective. NeuroRx, 2004. 1(2): p. 189-95.
[9] Mohammed Shabsog. Surrogate endpoints in EBM: what are the benefits and dangers? 4 September 2014. Available from https://s4be.cochrane.org/blog/2014/09/04/surrogate-endpoints-in-ebm-what-are-the-benefits-and-dangers/
[10] Seper Ekhtiari, Ryan P. Coughlin, Nicole Simunovic, et al. Surrogate Endpoints. Evidence-Based Surgery. Switzerland: Springer Nature; 26 March 2019; 85-92.
[11] Sarah Hand. Surrogate endpoints: how to choose the best one for your rare disease trial. 17 September 2020. Available from https://xtalks.com/surrogate-endpoints-how-to-choose-the-best-one-for-your-rare-disease-trial-2427/
[12] Fleming, T.R. and D.L. DeMets, Surrogate end points in clinical trials: are we being misled? Ann Intern Med, 1996. 125(7): p. 605-13.
[13] Wickstrom, K. and J. Moseley, Biomarkers and Surrogate Endpoints in Drug Development: A European Regulatory View. Invest Ophthalmol Vis Sci, 2017. 58(6): p. BIO27-BIO33.
[14] Wilson, K., et al., Duchenne and Becker Muscular Dystrophies: A Review of Animal Models, Clinical End Points, and Biomarker Quantification. Toxicol Pathol, 2017. 45(7): p. 961-976.
[15] Gilles Plourde. Validation of Surrogate endpoints for clinical trials. J of Pharmacol & Clin Res. 2018; 5(2): p. 555657.
[16] Fleming, T.R. and J.H. Powers, Biomarkers and surrogate endpoints in clinical trials. Stat Med, 2012. 31(25): p. 2973-84.
[17] Loeve, M., et al., Chest computed tomography: a validated surrogate endpoint of cystic fibrosis lung disease? Eur Respir J, 2013. 42(3): p. 844-57.
[18] Hunter, D.J., et al., A pathway and approach to biomarker validation and qualification for osteoarthritis clinical trials. Curr Drug Targets, 2010. 11(5): p. 536-45.
[19] Vokinger, K.N. and A.S. Kesselheim, Application of orphan drug designation to cancer treatments (2008-2017): a comprehensive and comparative analysis of the USA and EU. BMJ Open, 2019. 9(10): p. e028634.
[20] Kemp, R. and V. Prasad, Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med, 2017. 15(1): p. 134.
[21] Brandon Dyson. A guide to clinical trial endpoints. 25 January 2021. Available from https://www.tldrpharmacy.com/content/a-guide-to-clinical-trial-endpoints.
[22] Manju Bhaskar. The endpoint selection: a complex process in the clinical trials design. 22 July 2020. Available from https://cra-school.com/endpoint-selection-a-complex-process-in-clinical-trials/.
[23] McCoy, C.E., Understanding the Use of Composite Endpoints in Clinical Trials. West J Emerg Med, 2018. 19(4): p. 631-634.
*Corresponding author: C Fratazzi
BBCR Consulting Cambridge, MA, USA.
Copyright © 2022 Author(s) retain the copyright of this article. This article is published under the terms of the Creative Commons Attribution Liscense 4.0.
Let's Work Together
CONTACT US TO SCHEDULE A FREE CONSULTATIONBBCR is committed to meeting the challenges of sponsors and investors seeking a clear path to market.
